There is increasing interest in understanding the effects of early environmental adversities on the developing brain. Childhood maltreatment, including neglect and physical, sexual, and emotional abuse, is common in the United Kingdom, with pediatric prevalence rates of 9.8% for severe neglect, 6.9% for severe physical abuse, and 4.8% for sexual abuse (
1). Childhood adversities are significantly associated with first onsets of various psychiatric disorders, including mood and anxiety disorders (
2).
The psychopathological outcomes associated with childhood maltreatment may be mediated by the disruption of cognitive processes and their associated neural underpinnings (
3). Childhood maltreatment has been associated with various adverse cognitive consequences, such as low IQ and poor academic performance as well as impaired attention, inhibition, emotion, and reward processing (
4). Notably, cognitive control deficits have been reported in maltreated (
5,
6) and institutionalized children (
7,
8) as well as in adults who experienced childhood sexual abuse (
9).
Cognitive control, particularly the ability to monitor one’s ongoing performance and detect errors, is a key cognitive function critical to mature adaptive behavior (
10). Substantial improvement in cognitive control and error monitoring occurs from childhood to early adulthood; it is underpinned by progressively increasing fronto-cingulo-striatal activation with increasing age during this developmental period. In particular, dorsomedial frontal activation during error processing increases in a linear manner with age between childhood and adulthood (
11–
13).
Studies of error monitoring have focused mostly on the error-related negativity, an event-related potential (ERP) component associated with error detection localized to the medial frontal/anterior cingulate/supplementary motor area (
14). Enhanced error-related negativity has been associated with high sensitivity to punishment and hypervigilance (
15) and is typical for common comorbidities of childhood maltreatment, including depression and anxiety (
16). It is further suggested that environmental adversity and punitive parental behavior, which are often considered etiological factors for various internalizing disorders, may be linked to increases in error-related negativity, which are associated with these disorders (
17).
The ability to detect errors and adjust behavior accordingly may be particularly crucial in abusive settings, where mistakes are often associated with harsh punishment. Furthermore, maltreated children receive more negative evaluative feedback from their parents, which predisposes them to experience more shame when they fail on tasks (
18). Maltreated individuals also tend to avoid threat (
19) and exhibit heightened neural reactivity to threat-related faces (
20–
22); furthermore, their hypersensitivity to punishment is associated with increased risk taking to avoid potential punishments (
23). Thus, given that punishment and punitive parenting lead to lasting enhanced error-related negativity (
17,
24), persistent harsh punishment experiences in childhood may sensitize the abused child to errors and lead to an overactive error-monitoring system.
Childhood maltreatment is associated with significant volumetric differences in the lateral and ventromedial fronto-limbic areas and networks (
25,
26). Our recent meta-analysis (
26) showed that the most consistent gray matter deficits are in relatively late-developing inferior frontal and orbitofronto-limbic and temporal regions that mediate late-developing cognitive control and affect, respectively. However, relatively few functional MRI (fMRI) studies have been published in childhood maltreatment, and only three studies (
27–
29) have examined inhibitory networks. During successful inhibition, abused youths were found to have increased activation in the inferior, medial frontal, and anterior cingulate cortices relative to healthy subjects using the go/no-go (
27) and stop-change tasks (
28). However, in an adult study using the stop task (
29), a history of childhood maltreatment was not associated with changes in brain activation but was associated with decreased functional connectivity of the inferior frontal and dorsal anterior cingulate cortices.
In this study, we examined the association between severe childhood physical abuse and neural networks of inhibitory control and error processing in medication-naive, drug-free young people, using a challenging tracking stop task that ensures 50% inhibition failures and is thus optimally suited to test for error-detection networks. Sexual abuse was excluded because it has different effects on brain structure (
30) and different behavioral and psychiatric consequences (
31). To assess the specificity of the association with abuse, we included both a healthy comparison group and a psychiatric comparison group that was matched to the abused group on psychiatric comorbidities. We hypothesized that the abused group, relative to both comparison groups, would have increased activation in typical error-monitoring regions of the dorsomedial frontal cortex, including the anterior cingulate cortex and presupplementary and supplementary motor area (
32–
34) as well as in inferior frontal areas of inhibitory control (
27,
28).
Method
Participants
A total of 70 right-handed, medication-naive, drug-free, age-matched young people between 13 and 20 years old (23 in the childhood abuse group, 20 in the psychiatric comparison group, and 27 in the healthy comparison group) were initially assessed by a child psychiatrist using the Development and Well-Being Assessment (
35), which was designed to generate ICD-10 and DSM-IV psychiatric diagnoses. The Strengths and Difficulties Questionnaire (
36) and the Beck Depression Inventory (BDI) (
37) were used to provide symptom scores on psychopathology. IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (
38). The Childhood Trauma Questionnaire (
39) was used to measure the severity of childhood physical, sexual, and emotional abuse and physical and emotional neglect. Interviews using the Childhood Experience of Care and Abuse (
40) were further conducted to substantiate the information from the Childhood Trauma Questionnaire. Socioeconomic status was measured by two nonsensitive items (on housing tenure and room occupancy) from the Family Affluence Scale (
41).
Exclusion criteria for all participants were childhood sexual abuse, drug abuse, learning disability, neurological abnormalities, epilepsy, IQ <70, and MRI contraindications. Urine screening for recent drug use was conducted with 10-panel urine drug test integrated cups (T-Cup;
http://www.testfield.co.uk). Parental and participants’ informed consent or assent and approval from the local ethics committee were obtained.
The 23 participants who had experienced childhood physical abuse were recruited through social services and psychiatric clinics. They scored ≥13 on the physical abuse subscale of the Childhood Trauma Questionnaire, and the abuse history was corroborated by social service records and Childhood Experience of Care and Abuse interviews. Psychiatric comorbidities included posttraumatic stress disorder (PTSD), depression, anxiety, conduct disorder, and phobia. One participant was excluded because of MRI motion artifacts, leaving a final sample of 22 participants in the childhood abuse group.
The 20 participants in the psychiatric comparison group, who were matched with the childhood abuse group on psychiatric comorbidities but had no history of childhood maltreatment (they scored <8 for physical abuse, <9 for emotional abuse, <6 for sexual abuse, <10 for emotional neglect, and <8 for physical neglect) were recruited through psychiatric clinics and social services. PTSD patients experienced non-abuse-related trauma (e.g., experienced bullying, lived in Afghanistan during wartime, witnessed a murder, experienced a car accident, or experienced the death of a loved one). Three participants were excluded because of motion artifacts, leaving 17 participants in the psychiatric comparison group.
The 27 healthy comparison subjects, who had no history of psychiatric illness and childhood maltreatment, were recruited through advertisements in the same geographic areas of South London to ensure a similar socioeconomic background.
fMRI Paradigm: Stop Task
The stop task in a rapid, mixed-trial, event-related fMRI design was practiced by participants once before scanning. The visual tracking stop task requires withholding a motor response to a go stimulus when it is followed unpredictably by a stop signal (
12,
42,
43). The basic task is a choice reaction time task (left and right pointing arrows—go signals) with a mean interstimulus interval of 1.8 seconds (234 go trials). In 20% of trials, pseudorandomly interspersed, the go signals are followed (about 250 ms later) by arrows pointing upward (stop signals), and participants have to inhibit their motor responses (60 stop trials). A tracking algorithm changes the interval between go-signal and stop-signal onsets according to each participant’s inhibitory performance to ensure that the task is equally challenging for everyone and to provide 50% successful and 50% unsuccessful inhibition trials at every moment of the task (see Figure S1 in the
data supplement that accompanies the online edition of this article). Brain activation in the failed and successful stop trials is contrasted with the implicit baseline go trials. Participants completed two additional fMRI tasks (sustained attention and emotion processing), the results of which are not reported here.
Performance Data Analysis
Multiple analyses of variance (ANOVAs) were used to compare the main variables of stop task performance among the three groups, using SPSS, version 16 (SPSS, Inc., Chicago): stop-signal reaction time, mean reaction time to go trials, post-error reaction time, omission errors, and the probability of inhibition to stop trials. We used the Bonferroni adjustment for multiple comparisons.
fMRI Acquisition and Analysis
The fMRI acquisition procedures are described in the online data supplement.
Image preprocessing and whole-brain analyses were carried out using SPM8 (
www.fil.ion.ucl.ac.uk/spm). After preprocessing (see the
data supplement), data were analyzed within the framework of the general linear model. A first-level model was created for each participant, including regressors encoding failed stop and successful stop trials. Movement parameters from the realignment procedure were included in the model as regressors of no interest. For second-level (group) analyses, contrast images from the first-level analysis were used to conduct full factorial whole-brain analyses for each condition. Blood-oxygen-level-dependent (BOLD) responses are reported using a stringent cluster threshold of p<0.05, family-wise error rate corrected, and voxel threshold of p<0.001 for within-group activations for the two contrasts. Given the limited studies aimed at specifying brain differences in abused populations, and to control for the false positive rate (using p<0.05 family-wise error rate-corrected cluster statistics) while limiting potential type II errors, we chose an a priori cluster-forming threshold of p<0.01 for significant between-group differences.
Additionally, regions showing significant group differences were extracted using MarsBaR (
http://marsbar.sourceforge.net/) and defined using spherical masks with a radius of 6 mm around the peak coordinates for subsequent correlational analyses. These regions were selected to represent the main differences for confirmatory analyses on the influence of potential confounders such as IQ and task performance and for exploratory analyses examining the relationship with abuse measures.
To determine whether functional differences may be accounted for by anatomical variation, gray matter volumes of the regions showing group differences were compared among the three groups using total gray matter volume as a covariate.
Results
Participant Characteristics
The groups did not differ significantly in age, gender, ethnicity, and socioeconomic status but differed on IQ, as expected (
Table 1). Since childhood maltreatment is associated with lower IQ (
44,
45), artificially matching groups on IQ is inappropriate, as it creates unrepresentative groups: either the abused group will have a higher mean IQ than the abused population, or the comparison group will have a mean IQ below normative expectations (
46). Also, it is misguided to control for IQ differences by covarying IQ when groups are not randomly selected and the covariate is a pre-existing group difference, as analysis of covariance (ANCOVA) would lead to potentially spurious results (
46,
47). The data are therefore presented without matching or covarying IQ. However, to explore and rule out any potential influence of IQ, additional reanalysis of a subsample of IQ-matched participants, an ANCOVA covarying for IQ, and a correlational analysis of IQ with brain activation and performance measures were conducted (additional confirmatory analyses).
Although we selected participants with severe childhood physical abuse, they also experienced marked or severe childhood emotional abuse and neglect (
Table 1), which typically co-occur with physical abuse, and hence they are a representative group of the abused population (
48,
49).
The healthy comparison group scored significantly lower than the childhood abuse group on the BDI and all the difficulties subscales of the Strengths and Difficulties Questionnaire, and lower than the psychiatric comparison group on the BDI and on the emotional problems and hyperactivity/inattention subscales of the Strengths and Difficulties Questionnaire. The childhood abuse group scored significantly higher than the psychiatric comparison group on the conduct and peer problems subscales of the Strengths and Difficulties Questionnaire (
Table 1).
Task Performance
Mean performance values are reported in
Table 2. The probability of inhibition was about 50% in all participants, with no significant group differences, showing that the task algorithm was successful.
The groups differed significantly on mean reaction time to go trials and post-error reaction time, but not on stop-signal reaction time. Post hoc analyses showed that the childhood abuse group had significantly slower responses on both measures than the healthy comparison group (p<0.05).
Brain Activation
Motion.
Multivariate ANOVAs showed no significant group differences in mean or maximum translation or in rotation parameters.
Failed stop-go contrast.
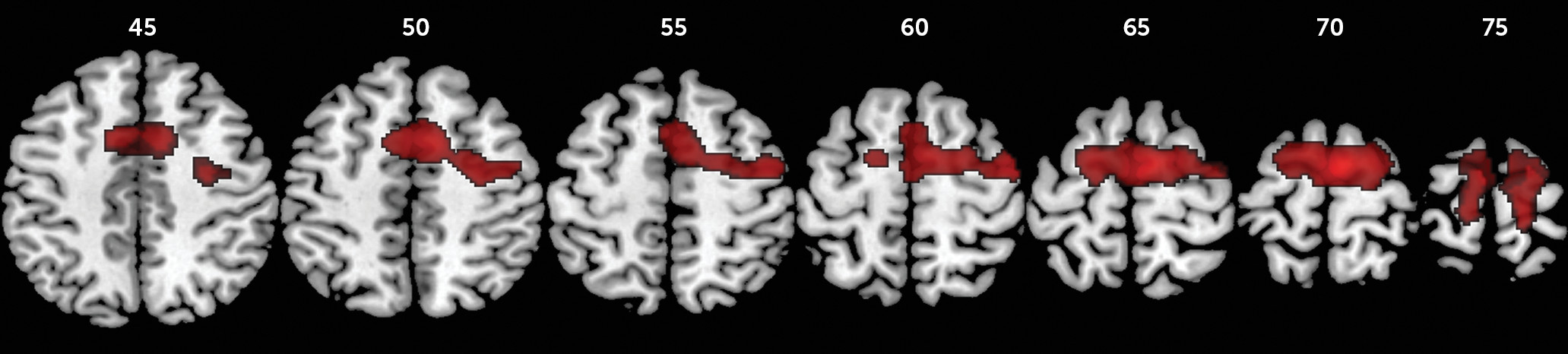
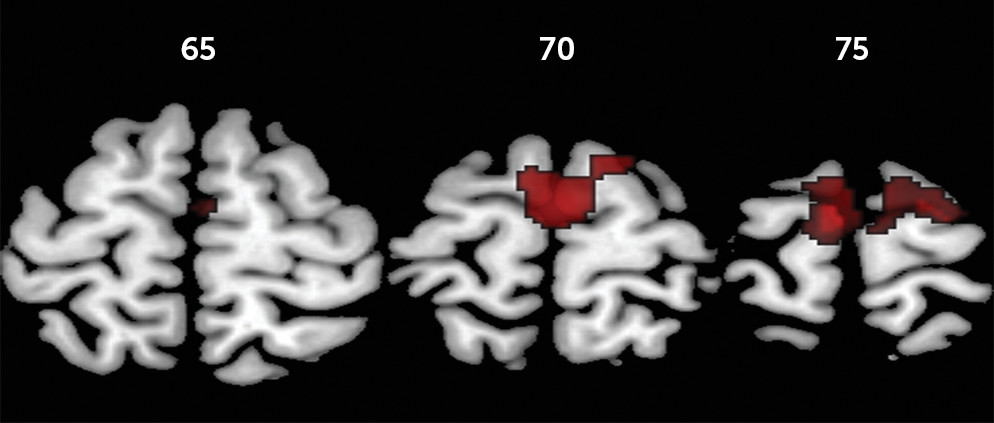
For failed inhibition, ANOVA showed a significant group effect in a large cluster comprising the left and right presupplementary and supplementary motor area, dorsal anterior cingulate cortex, and superior frontal gyri and the left paracentral lobule. Post hoc comparisons showed that the childhood abuse group had increased activation in these regions relative to the healthy comparison group and in a subcluster within the supplementary motor area bilaterally relative to the psychiatric comparison group. The psychiatric and healthy comparison groups did not differ significantly from each other. Given our hypothesis that the childhood abuse group would have increased error-related activation relative to both comparison groups, further planned group comparisons showed that the childhood abuse group had increased activation in a slightly larger cluster of the above-mentioned regions and additionally in the left and right precentral, right postcentral, and middle frontal gyri relative to the healthy comparison group and in the supplementary motor area bilaterally compared with the psychiatric comparison group (
Table 3,
Figures 1 and
2).
For the cluster of significant group differences, a spherical mask with a radius of 6 mm around the peak voxel (−2, −4, 74) was defined, and BOLD response was extracted for correlational analyses with IQ and the main performance measures (go and post-error reaction times) within each group, and with abuse measures within the childhood abuse group only (see Table S3 in the online data supplement). There were no significant correlations.
Data on within-group brain activations are presented in the data supplement.
Additional confirmatory analyses.
Given that the childhood abuse group had a significantly lower mean IQ than the healthy comparison group, data were reanalyzed 1) using an IQ-matched subsample (22 in the childhood abuse group, 17 in the psychiatric comparison group, and 17 in the healthy comparison group) and 2) covarying for IQ (see Figures S4 and S5 in the data supplement). All main findings remained significant. Also, within each group, IQ did not correlate significantly with BOLD response or with performance measures (see Table S3 in the data supplement). It is therefore unlikely that IQ differences explain the findings.
Since the childhood abuse participants responded slower on go trials than did healthy comparison participants, we reanalyzed the data for a subsample (22 in the childhood abuse group and 23 in the healthy comparison group) matched on mean go reaction time. The main findings remained significant (see Figure S6 in the
data supplement). There were also no significant group differences between healthy individuals with high versus low mean go reaction times (median split at 475 ms). Moreover, the main findings also remained significant when we covaried for both go and post-error reaction times (see Figure S7 in the
data supplement). Thus, performance differences were unlikely to confound the findings.
Next, we examined a subsample (16 in the childhood abuse group, 17 in the psychiatric comparison group, and 17 in the healthy comparison group) matched on psychopathology symptoms, using the total difficulties score on the Strengths and Difficulties Questionnaire. The group-difference findings remained significant, except in the left dorsal anterior cingulate cortex, possibly because of loss of power (see Figure S8 in the data supplement). Furthermore, there were no significant group differences when the 25 patients with PTSD were compared with the 27 healthy comparison participants or with the 41 participants who did not have PTSD, suggesting that the functional abnormalities are related to abuse rather than PTSD.
Additionally, the findings remained significant when we examined subsamples of the childhood abuse group and the psychiatric comparison group matched on the conduct and peer problem subscales of the Strengths and Difficulties Questionnaire (see Figures S9 and S10 in the data supplement). Hence, the brain activation differences are related to abuse rather than to differences in externalizing symptoms.
Finally, the data were reanalyzed covarying for gender, ethnicity, and age (see Figure S11 in the data supplement). The main findings remained significant.
Successful stop-go contrast.
For successful inhibition, there were no significant group differences in activation (see the data supplement for within-group activations).
Brain Volume Differences
There were no significant group differences in total gray and white matter or medial frontal brain volumes (
Table 4).
Discussion
To our knowledge, this is the first fMRI study to examine the association between severe childhood abuse and error-related brain activation in medication-naive, drug-free young people. The childhood abuse participants had slower go and post-error reaction times than did healthy comparison subjects but showed no abnormalities on the inhibition measure. As hypothesized, the childhood abuse participants, relative to the healthy comparison subjects, exhibited significantly increased activation in typical error-monitoring regions of the dorsomedial frontal cortex, including the presupplementary and supplementary motor area and the dorsal anterior cingulate and superior frontal cortices. Furthermore, a smaller cluster within the supplementary motor area was significantly more activated in the childhood abuse group than in the psychiatric comparison group, who did not differ significantly from the healthy comparison group. No significant group differences in activation were observed during successful inhibition, suggesting that the functional abnormalities were specific to error processing. Furthermore, the main findings remained significant when we controlled for IQ, task performance, PTSD, and psychopathology/externalizing symptoms and were not confounded by differences in underlying brain anatomy. Thus, the functional abnormalities appeared to be specifically related to the abuse experience.
The dorsomedial frontal cortex is the key region for error processing, as shown in meta-analyses in which this area, including the dorsal anterior cingulate and presupplementary and supplementary motor area, is implicated in the detection of response errors and negative feedback that engages regulatory processes in the lateral prefrontal cortex to implement performance adjustments (
33). The presupplementary and supplementary motor area and anterior cingulate are typical regions of error processing/performance monitoring in healthy adults (
11,
12,
34,
42,
43,
50) and children (
11,
12,
42,
51) on the same or similar fMRI stop paradigms.
The supplementary motor area, which rapidly evaluates successful and erroneous actions, is known to be extensively involved in the assessment of ongoing actions (
52) and plays a leading role in the error-monitoring system (
32). Notably, childhood abuse participants had greater error-related activation within the supplementary motor area cluster compared with their psychiatric comparison counterparts, suggesting that the hyperactivation of this key error-processing region may be abuse specific.
All findings remained when we controlled for IQ, performance differences, and psychopathology symptoms, suggesting that the abnormalities are abuse specific. Also, the findings were not confounded by anatomical abnormalities in the medial frontal cortex, indicating that deficits were specific to the function rather than the structure of this region.
Nevertheless, we did not find significant correlations between functional abnormalities and abuse measures. This may be due to lack of power, or perhaps the correlations with the imaging data are related to transient activity beyond the temporal resolution of fMRI for accurate quantification.
The childhood abuse group demonstrated normal inhibitory capacity, which is consistent with previous performance findings (
27,
29). The negative group difference finding in inhibitory activation is consistent with a previous fMRI study that used the same stop-signal paradigm (
29). Although two other studies found impaired inhibitory activation, they used the go/no-go (
27) and stop-change (
28) tasks and recruited youths who experienced early deprivation (
28), posttraumatic stress symptoms, and childhood trauma such as sexual abuse and witnessing violence (
27), which were not included in our study. Hence, the findings are not directly comparable, and further studies are needed to examine the integrity of inhibitory networks in youths exposed to different types of maltreatment.
We speculate that the increased sensitivity to errors as expressed in longer post-error reaction time and increased activation in the supplementary motor area in the abused young people relative to age-matched healthy and psychiatric comparison subjects could be due to the constant need to monitor their actions to avoid potential painful mistakes. This hypothesis would be in line with evidence that environmental adversity such as punitive parental behaviors is associated with enhanced error-related negativity in ERP studies, which is related to hypersensitivity to punishment, hypervigilance (
15), and comorbid conditions typically associated with childhood maltreatment, such as depression and anxiety (
16). The findings may be the cognitive counterpart of evidence from the emotional domain that maltreated individuals tend to avoid threat (
19) and exhibit heightened neural reactivity to threat-related faces (
20–
22). Thus, we postulate that persistent harsh punishments in childhood may sensitize the abused child to errors and lead to an overactive error-monitoring system.
Among the strengths of this study were that all participants were medication-naive, they were drug free, and their abuse experience was carefully assessed and corroborated by social service records. Also, we included a psychiatric comparison group to determine the specificity of abuse in our findings. The inclusion of a group of abused young people without any psychiatric disorders would have provided a more robust means of determining abuse-specific deficits; however, such a “pure” abused group would not be representative of the general abused population, as high abuse severity is typically associated with psychiatric comorbidities. It is unclear to what extent pubertal development, malnutrition, and prenatal drug exposure may have influenced the findings. The socioeconomic status measure we used is limited, as it does not include information on parents’ income and education; however, youths often have difficulties in reporting this information (
41). Although we excluded childhood sexual abuse because it has been shown to differ in many aspects from physical abuse (
31) and includes a distinctive effect on the somatosensory cortex (
30), it is unrealistic to separate severe physical abuse from typically co-occurring emotional abuse and neglect (
48,
53).
In summary, we found that young people who had experienced severe childhood abuse had greater error-related activation in the anterior cingulate cortex and the supplementary motor area than healthy subjects. In particular, the increased activation in the supplementary motor area was abuse-specific. The childhood abuse group showed no abnormal inhibitory activation relative to the comparison groups. Hence, abuse victims may develop a greater sensitivity to errors as a form of adaptation to an environment in which errors frequently predict the occurrence of abuse. These findings represent a first step toward the delineation of abuse-specific neurofunctional abnormalities such as hyperactive error processing, which may lead to the development of specific treatment strategies for victims of childhood abuse.
Acknowledgments
The authors thank Dr. Kaylita Chantiluke and Miss Sinead King for their assistance with data collection.