Bipolar disorder is an episodic and debilitating mood disorder that affects approximately 1% of the general population (
1,
2). Extensive work has been done to understand the role of genes and regulation in the bipolar disorder brain. Candidate gene and genome-wide microarray expression analyses of postmortem human brains have shown that transcriptional dysregulation plays a role in the etiology of bipolar disorder (for a review, see reference
3). Some notable genes have been identified through these studies (
4,
5); however, very few passed corrections for multiple testing, and findings from these studies have largely not been replicated (
3,
5,
6). RNA sequencing, a technique that takes advantage of the recent development of high-throughput sequencing technologies, offers a number of advantages in comparison to previous methodologies, such as microarray studies, in that it provides direct estimates of transcript abundance, as well as nucleotide-level sequence. Differential expression can be measured both at the gene and transcript levels, thus providing unbiased and unparalleled evidence for novel RNAs (
7) and regulatory mechanisms such as alternative splicing (
8), which are not well represented on microarrays. Transcriptome analysis using RNA sequencing in bipolar disorder is timely and important, not only given the power of the technology but also given the need for greater understanding of the bipolar disorder pathophysiology and development of effective treatment options.
In order to understand the role that coding and noncoding RNAs play in brain regulation and how their potential dysregulation could affect brain function and ultimately onset of bipolar disorder, we investigated gene expression changes in postmortem brain tissue from bipolar disorder cases using RNA sequencing. While the precise bipolar disorder neuroanatomical circuits are not exactly known and there are data supporting the involvement of diverse brain regions, there is strong support for the role of the anterior cingulate cortex in the regulation of ideo-affective and mood functions and thus in the neurobiology of bipolar disorder (
9,
10). Consequently, we focused this postmortem expression study on this region and found a global pattern of downregulation. Furthermore, we identified several differentially expressed genes and followed up these findings with an in vitro study that showed the mood stabilizers lithium, carbamazepine, and valproate to modulate the expression of these transcripts. By using RNA sequencing we hope to have achieved a more comprehensive level of understanding of the bipolar disorder brain and shed important light on the dysregulated mechanisms, as well as the potential implications for treatment.
Discussion
In this study, we investigated the transcriptome of individuals with bipolar disorder in postmortem brains from the anterior cingulate cortex (Brodmann’s area 24) using RNA sequencing. Extensive evidence from microarray and candidate gene studies has demonstrated the role of transcriptional dysregulation in the etiology of bipolar disorder (
3). Furthermore, accumulating evidence is starting to point to the key involvement of as-yet uncharacterized noncoding RNAs, in addition to the more commonly studied protein-coding RNAs in psychiatric disorders.
We showed excellent validation of our methods, both at the bioinformatics level across two different pipelines and at the molecular level with quantitative real-time PCR. Furthermore, we replicated our top findings in an external cohort consisting of anterior cingulate cortex samples from 61 postmortem brains from the Stanley Neuropathology Consortium Integrative Database collection, for which transcriptome sequencing findings were recently described in a study conducted by Zhao et al. (
22). By-and-large, we found a strikingly prominent global downregulation, with all differentially expressed transcripts that passed multiple testing corrections (false discovery rate ≤0.05) being downregulated, as well as an enrichment of downregulated genes among the top 100 genes ranked by p value and a much more consistent expression pattern across subjects in downregulated genes compared with upregulated ones. The top gene identified by both gene-level bioinformatics pipelines and the isoform-level analysis was
B3GALT2 (UDP-Gal:betaGlcNAc beta 1,3-galactosyltransferase, polypeptide 2) (
28). Quantitative real-time PCR validation in our cohort was supportive of this finding, and in the Stanley Neuropathology Consortium Integrative Database replication cohort this gene was also downregulated, though at a suggestive significance level. This gene is a member of the beta−1,3-galactosyltransferase (beta3GalT) family, which encodes type-II membrane-bound glycoproteins. Though very little is known about
B3GALT2 and associations with bipolar disorder have not yet been documented, genes from this family have been shown to be primarily brain-expressed in the mouse (
29). Furthermore, B3galt2 knock-out mice have been shown to have decreased anxiety-related response and increased hyperactivity through the Open Field test, as well as decreased coping response through the Tail Suspension test, indicating a possible depression-like phenotype (
30). It is also worth noting that Akula et al. (
23), who conducted a bipolar disorder RNA sequencing study in the dorsolateral prefrontal cortex, also detected this gene to be downregulated.
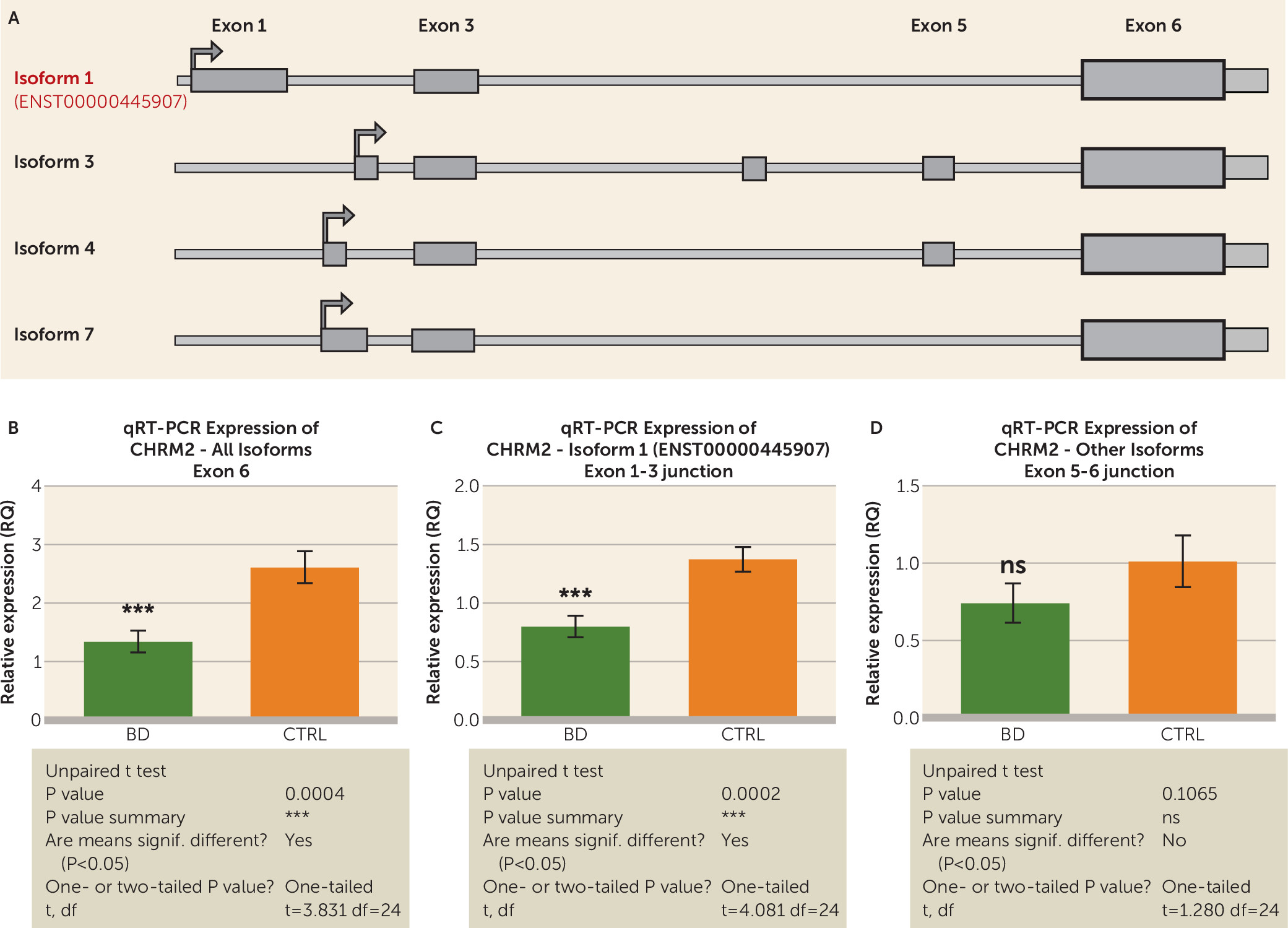
One of our most interesting findings was the differential expression of G protein-coupled receptors, as suggested by the gene-set enrichment analysis that indicated an enrichment of G protein-coupled receptor pathways among the differentially expressed genes, supported by the fact that three of the top differentially expressed genes,
SSTR2 (somatostatin receptor 2),
CHRM2 (cholinergic receptor, muscarinic 2), and
RXFP1 (relaxin/insulin-like family peptide receptor 1), are class A G protein-coupled receptors. These noted drug targets in many disorders, including those afflicting the brain (
31), have previously been linked to mood disorders, including bipolar disorder (
32). Of the top three G protein-coupled receptors that we have identified in this study, only
CHRM2, a muscarinic receptor defined by the binding of acetylcholine and involved in adenylate cyclase inhibition, phosphoinositide degeneration, and potassium channel mediation, has been previously linked to bipolar disorder (
33,
34).
SSTR2 mRNA levels have been shown to be decreased in the prefrontal cortices of schizophrenia patients (
35) and to decrease in response to stress in animal models (
36). Interestingly, one recent study by Tomita et al. (
32) that focused specifically on microarray profiling G protein-coupled receptor expression in psychiatric postmortem brains found
SST (somatostatin) to be one of the top dysregulated genes in the anterior cingulate cortex of bipolar disorder patients. The specific G protein-coupled receptors that emerged in the top of our study were not mentioned in this study, though there was a great deal of overlap in the affected pathways, including inositol polyphosphate phosphatases, neuropeptide Y genes, and proteinphosphatase 1 genes (
32). Our study, along with others, suggests that G protein-coupled receptors as a whole should be further investigated in bipolar disorder.
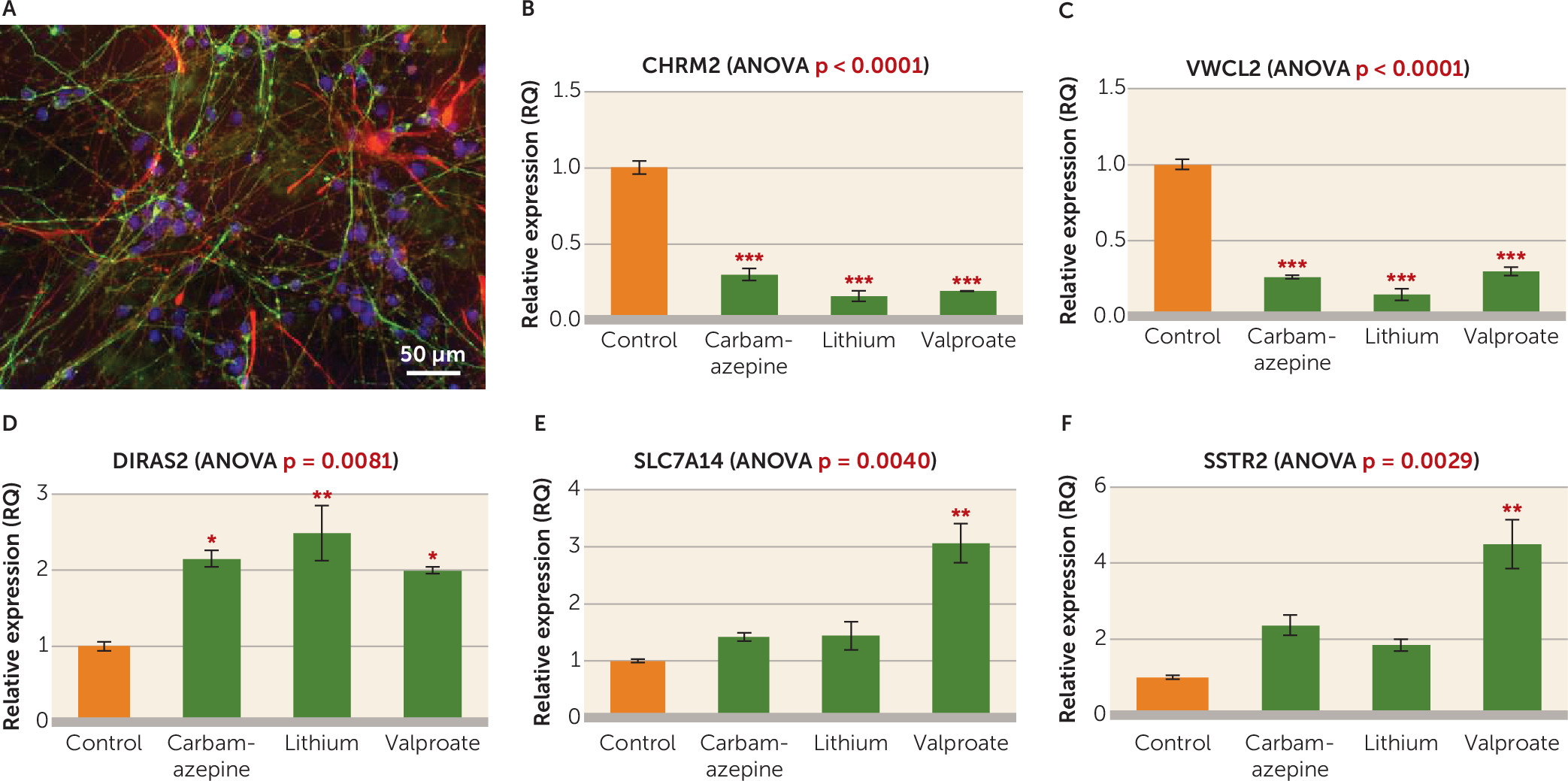
In order to further explore the role of the identified dysregulated genes, we investigated the effect of mood stabilizers lithium, valproate, and carbamazepine on the expression of the top differentially expressed genes through an in vitro chronic treatment experiment in cultured neural progenitor cells. These drugs were selected based on a long-standing history of documented efficacy in the clinical treatment of bipolar disorder (
1,
2). Since the majority of differentially expressed transcripts were significantly downregulated in the bipolar disorder brain, we were interested in the possibility that the expression of these genes would be upregulated by mood stabilizers.
DIRAS2 was upregulated by all the three drugs tested, while
SSTR2 and
SLC7A14 were significantly upregulated by valproate only. Very little is known about the implication of
DIRAS2 (DIRAS family, GTP-binding RAS-like 2) in the brain, as it has only been studied in adult attention deficit hyperactivity disorder (
37). Neither
SLC7A14 (solute carrier family 7, member 14) nor
SSTR2 has previously been investigated in bipolar disorder or valproate treatment, but the latter is a G protein-coupled receptor belonging to class 4A that has been implicated in adaptive response to stress (
36,
38). Furthermore, other somatostatins have been linked to bipolar disorder genetics (
39–
41). Expression of
CHRM2 and
VWC2L was decreased by all three drugs, which is unexpected considering that they were also downregulated in the bipolar disorder brains. There are different possible explanations for these findings. Among these, it is possible that some of the brain expression differences observed were compensatory, thus in a similar direction as those observed with action of therapeutic drugs. Similarly, it is possible that substance and past treatment exposure of the bipolar disorder cases may explain some of our significant findings, including
CHRM2 and
VWC2L. While encouraging, our results provide but the first steps in the attempt to elucidate how commonly prescribed mood stabilizers may influence the expression of genes dysregulated in the bipolar disorder brain.
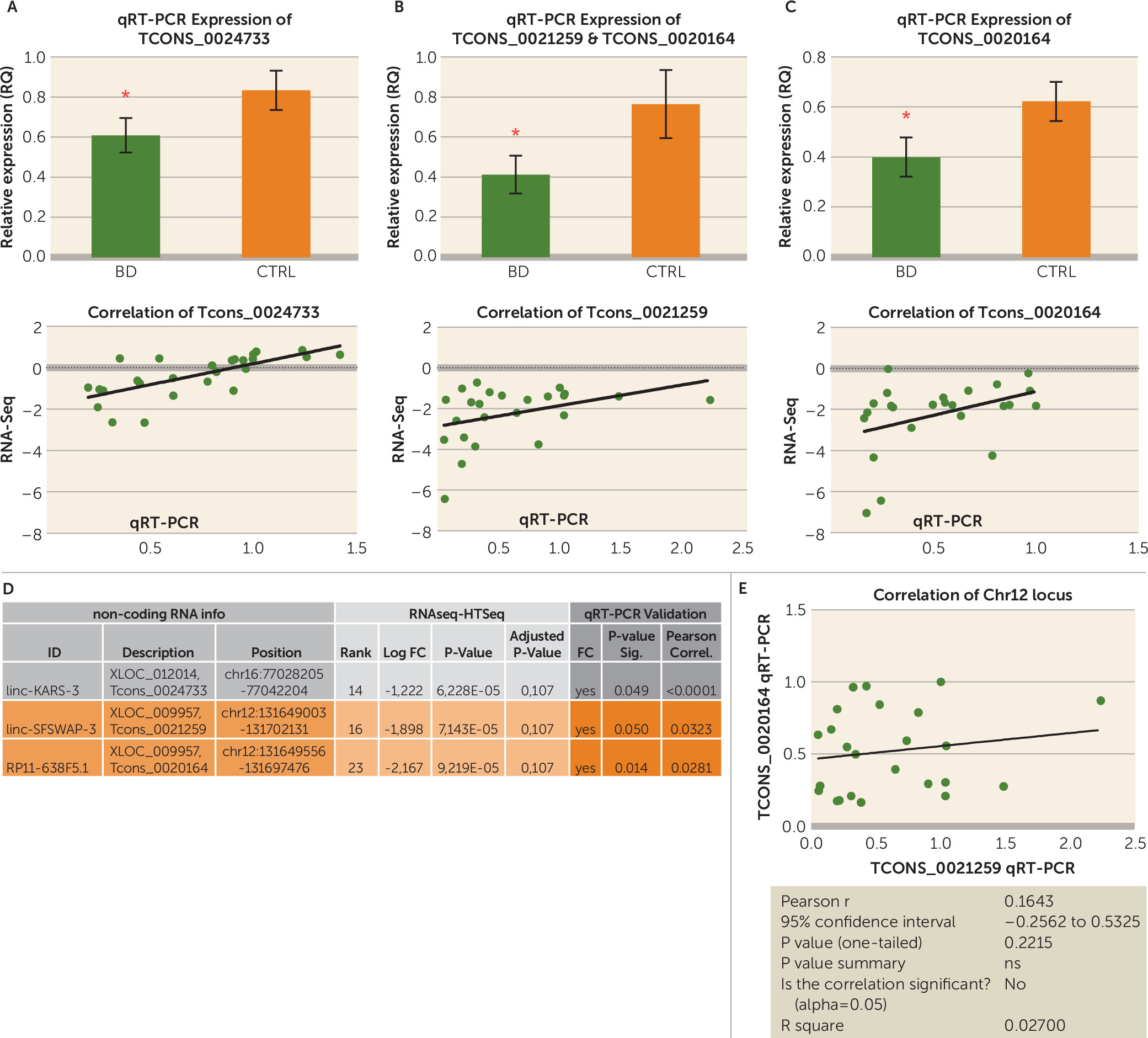
Finally, to our knowledge, this is the first study in psychiatry that used ribosomal depletion in the preparation of RNA sequencing libraries and thus can quantify all classes of RNA of 100 base pairs or longer, regardless of their possession of a poly-A tail. Furthermore, since we used a very high coverage, we were able to detect some very lowly expressed transcripts, including lincRNAs, antisense RNAs, small nuclear RNAs, and other noncoding classes that are not as abundant as protein-coding transcripts. This analysis is of interest because more and more reports have emerged in the last few years documenting the importance of noncoding RNAs in normal brain development, maintenance, and aging (
42–
44), as well as a variety of conditions including neurodevelopmental disorders such as autism (
45,
46). Though they did not pass multiple testing corrections, the top three (ranked by p value) most significant lincRNAs were significantly downregulated when validated using quantitative real-time PCR, suggesting that these lincRNAs should be further investigated in bipolar disorder. Unfortunately, we have no information from the literature to help us understand how dysregulation of these lincRNAs could be connected to the expression of the coding genes identified. Since the exploration of noncoding RNA species is still in its infancy, undoubtedly computational tools will improve along with our understanding of these RNA classes, allowing us to extract even more valuable knowledge. Further work is warranted to fully exploit the abundance of information collected with total transcriptome sequencing analysis.