Considerable evidence supports the efficacy of light therapy for winter seasonal affective disorder (SAD) (
1,
2). Clinical practice guidelines recommend daily light therapy from the first symptom each fall or winter until spontaneous remission in spring or summer (
3). Investigation of alternative treatments is warranted given the substantial minority (47%) of SAD patients who do not achieve remission with light therapy (
1).
We previously developed and tested a group cognitive-behavioral therapy adapted for SAD (CBT-SAD) (
4) in an initial feasibility test (
5) and a controlled randomized clinical trial (
6). These preliminary studies, including 84 adults with SAD, found comparable and large symptom improvements in patients receiving CBT-SAD and those receiving light therapy, with both groups showing larger benefits than patients in a concurrent wait-list control condition (
6). To more definitively assess CBT-SAD’s efficacy, the current trial was a larger head-to-head comparison of CBT-SAD and light therapy with stronger ecologic validity, through use of community therapists to facilitate CBT-SAD and relaxed inclusion/exclusion criteria to allow for comorbid psychopathology and stable antidepressant medications. This trial was designed as a superiority study, powered to detect large, clinically meaningful differences between CBT-SAD and light therapy in recurrence (the primary outcome) over 2 years following the end of treatment (
7). Here we examine the first wave of data in a secondary aim to estimate the difference in efficacy between CBT-SAD and light therapy regarding depressive symptom severity and remission status at treatment endpoint. We hypothesized that observed differences between treatments would be very small, neither statistically significant nor clinically meaningful.
Method
Design and Power
Our complete protocol is archived elsewhere (
7). Currently depressed SAD patients were randomly assigned to 6 weeks of CBT-SAD or light therapy in a concurrent two-arm design. This study took place at the Mood and Seasonality Laboratory at the University of Vermont and was approved by the university’s institutional review board. This trial was powered to test the primary hypothesis that CBT-SAD would be superior to light therapy in depression recurrences following treatment of the index episode, whereas these secondary analyses compared the treatments at treatment endpoint. However, with these sample sizes (CBT-SAD, N=88; light therapy, N=89), there was 80% power to detect differences of ≥3.0 in posttreatment scores on the Structured Interview Guide for the Hamilton Rating Scale for Depression–Seasonal Affective Disorder Version (SIGH-SAD) (
8) and a 0.21 difference in the proportions of patients in remission.
Participants
Community volunteers, age 18 or older, were recruited through local media advertisements and referrals from health clinics in the fall and winter months. Inclusion criteria were 1) fulfillment of the DSM-IV-TR criteria for major depression, recurrent, with a seasonal pattern, 2) fulfillment of the SIGH-SAD criteria (
8) for a current SAD episode, and 3) no or stable use of antidepressants (i.e., a consistent dose of the same medication maintained for at least the past 4 weeks with no plans for change). Exclusion criteria included 1) current light therapy or psychotherapy for depression, 2) prior light therapy or CBT for SAD, 3) a comorbid axis I disorder requiring immediate treatment (e.g., psychotic disorder, substance abuse/dependence, bipolar disorder), 4) acute and serious suicidal intent, 5) positive laboratory findings for hypothyroidism at medical workup, and 6) plans for a vacation or absence for more than a week through March.
Screening and Enrollment
Recruitment occurred between September and mid-January for 6 consecutive years beginning in 2006. Following a brief telephone screening, potentially eligible respondents were invited to review the informed consent form. Those who consented came for in-person diagnostic interviews using the Structured Clinical Interview for DSM-IV Axis I Disorders–Clinician Version (SCID) (
9), administered by the principal investigator (K.J.R.) or a trained clinical graduate student. Individuals meeting the SCID criteria for recurrent major depression with a seasonal pattern were interviewed with the SIGH-SAD. A SIGH-SAD score of ≥20 (including a score on the atypical symptom subscale of ≥5) was the marker of current depression. If the threshold was not reached, individuals were reassessed every other week. After meeting the SIGH-SAD criteria, individuals underwent a medical workup at the university’s Clinical Research Center in the College of Medicine, including a thyroid panel to rule out hypothyroidism.
Randomization
Random assignment to CBT-SAD or light therapy was based on permuted random blocks of size 4 and 6, stratified by sex, comorbid axis I diagnosis (present/absent), and current antidepressant medication status (positive/negative). All investigators except the project statistician were blinded to the randomization schedule. The project coordinator randomly assigned participants on a rolling admission each fall and winter when the interventions were concurrently implemented. The 6-week treatment phase commenced no later than the first week of February to ensure completion before spring, when spontaneous remission occurs.
Treatments
Light Therapy.
A scripted-protocol instructional session included demonstration of device assembly and positioning and review of the treatment rationale, instructions, and possible side effects. We used the 23×15½×3¼-in. SunRay (SunBox Company, Gaithersburg, Md.), which emits 10,000 lux of cool-white fluorescent light through an ultraviolet filter. Given that morning is more effective than evening for light therapy (
1), the starting dose was 30 minutes immediately upon awakening (
10). After week 1, the following treatment algorithm was used to maximize response and reduce side effects. Increasing the daily duration, incrementally by 15 minutes per week up to a maximum of 2 hours per day, was indicated by an insufficient response, defined as a SIGH-SAD score reduction less than 30% at week 1 or less than 50% at week 2 or as not fulfilling the SIGH-SAD remission criteria at week 3 or beyond. In the case of significant side effects, duration was decreased in 15-minute decrements to a minimum of 30 minutes per day. Severe side effects (e.g., migraines) warranted a 1-day hiatus from light therapy, with resumption the following day at 50% of the prescribed duration and subsequent increases to a tolerable level. In the event of early awakenings and/or early evening sleepiness, morning light was reduced and/or a daily evening light session was added, beginning with 10 minutes and adding more as needed. Side effects were monitored weekly through self-report diaries. Once per week, the principal investigator and a chronobiological psychiatrist with light therapy expertise (T.T.P.) reviewed each light therapy subject’s file according to this algorithm, and the principal investigator conveyed any recommended clinical adjustments to subjects over the telephone within 24 hours. After the 6 weeks of monitored light therapy, we encouraged participants to continue daily light therapy until their typical time of spontaneous remission, and we collected the devices from them in May.
CBT-SAD
CBT-SAD (
4) is an adaptation of traditional cognitive therapy for depression (
11) to specifically target SAD. CBT-SAD uses behavioral activation and cognitive restructuring to improve coping with winter, thereby alleviating depression and fortifying against relapse and recurrence. Identifying and scheduling pleasant events is used to combat winter anhedonia. In addition to targeting typical depressive thought content, some cognitive restructuring challenges negative thoughts related to the winter season (e.g., focus on darkness or winter weather). The protocol concludes with a personalized relapse-prevention plan involving early identification of negative anticipatory thoughts about winter and SAD-related behavior changes as signals to implement CBT skills to prevent recurrence. Unlike cognitive therapy for depression (
11) (twenty 50-minute sessions over 16 weeks), SAD necessitates a condensed schedule to accommodate completion before spring (two 90-minute sessions per week over 6 weeks). Sessions were audiotaped to assess treatment adherence.
Training and Supervision of Community Therapists
CBT-SAD was conducted in small closed groups of four to eight participants, with each group led by a licensed Ph.D.-level psychologist (the principal investigator or one of two community therapists) and a clinical psychology graduate student co-therapist. The community therapists had a minimum of 4 years of postdoctoral practice, and both had prior clinical experience treating depression with CBT. Before facilitating groups, the therapists met with the principal investigator to review the protocol in detail. For each therapist’s first group, the principal investigator listened to audiotapes of each session and then met weekly with the therapist to review the prior two sessions and plan the next two. After the closely supervised first group, the principal investigator reviewed at least two more audiotaped sessions per group and provided 1.5 hours of weekly supervision with each therapist. The therapists were compensated for their time in training and supervision at the standard hourly clinical rate in Vermont and for their time administering CBT-SAD at the standard per-patient group therapy rate.
Treatment Integrity Measure
To assess treatment adherence across sessions and therapists, we previously adapted the National Institute of Mental Health’s Collaborative Study Psychotherapy Rating Scale (
12,
13) to assess therapist behaviors in CBT-SAD and light therapy (
6). A random sample (25%) of sessions varying across conditions and, for CBT-SAD, across groups, session numbers, and therapists, were independently rated by two trained clinical psychology graduate students, blind to condition and session number.
Outcome Measures
The 29-item SIGH-SAD (
8) was administered by a blind rater before treatment, at weeks 1–5, and after treatment. SIGH-SAD-derived outcomes included total score (range=0–90), scores on its component subscales (the 21-item Hamilton Rating Scale for Depression [HAM-D] and the 8-item atypical symptom subscale), and remission status after treatment. Either of the following was classified as a remission (
14): 1) pre- to posttreatment reduction in SIGH-SAD score of ≥50% plus a HAM-D score of ≤7 plus an atypical symptom score of ≤7 or 2) HAM-D score of ≤2 plus an atypical symptom score of ≤10. SIGH-SAD interviews were audio-recorded and rated by a second blind rater. The intraclass correlations for interrater reliability were 0.923 before pretreatment; 0.958, 0.965, 0.950, 0.967, and 0.962 for weeks 1–5; and 0.961 after treatment.
The Beck Depression Inventory—Second Edition (BDI-II) (
15), a 21-item self-report measure of depressive symptom severity, was administered before treatment, at week 3 (midtreatment), and after treatment. A BDI-II score of ≤8 was used as a marker of remission, as in our prior trials (
5,
6,
16).
Statistical Analyses
To examine change in depression severity on the SIGH-SAD across the 6 weeks of treatment, we fitted mixed-effects regression models with treatment (CBT-SAD or light therapy), time (pretreatment, weeks 1–5, and posttreatment), and their interaction as fixed effects and subject as a random effect, using all available data. Analogous regression models were fitted to BDI-II scores at pre-, mid-, and posttreatment. Mean values for treatment conditions and time points were estimated as the least-squares means. Secondary analyses examined potential treatment moderators (sex, age, race, pretreatment depression severity, comorbidity status, antidepressant medication status) by entering each into the regression models as a fixed effect, together with its 2- and 3-way interactions with treatment and time. The proportions of participants in remission after treatment in the CBT-SAD and light therapy groups were compared by using a Pearson’s chi-square test. For all tests, p values less than 0.05 were considered statistically significant. Mixed-effects regression models were also used to assess the effects of therapist and CBT-SAD group membership on posttreatment depression scores.
Results
Participant Characteristics
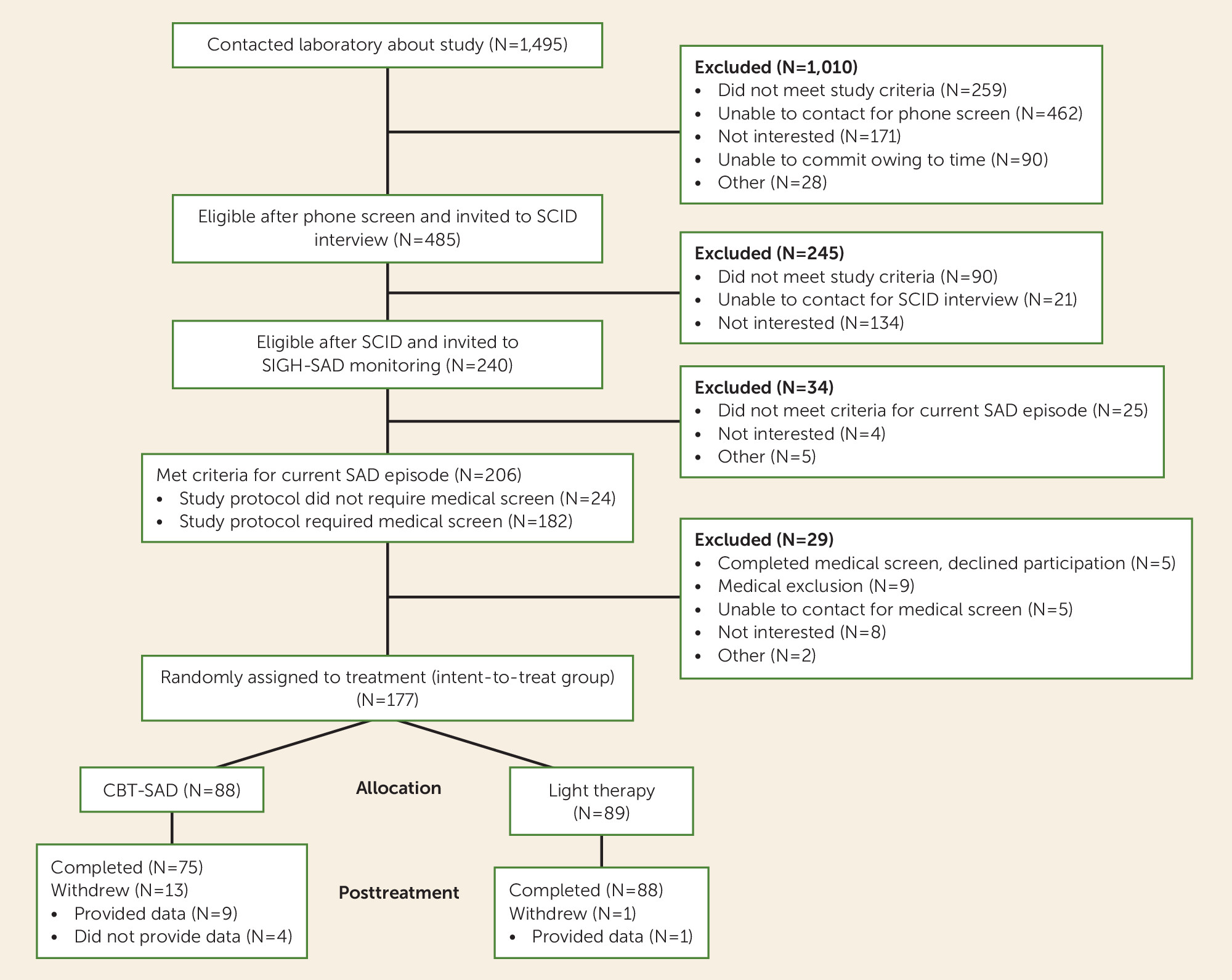
Figure 1 illustrates participant flow, beginning with the initial pool of individuals who contacted us about the trial and progressing through all successive screening phases, randomization, and treatment endpoint.
The intent-to-treat sample included 177 fully eligible patients who were randomly assigned to CBT-SAD (N=88) and light therapy (N=89). None was withdrawn because of adverse effects, and no harmful or unintended effects were observed in either treatment group. However, one participant (out of 89, 1.1%) voluntarily withdrew from light therapy, and 13 of 88 participants (14.7%) voluntarily withdrew from CBT-SAD. Efforts were made to obtain data from all withdrawn participants. Missing data were minimal; 173 patients (97.7%) provided SIGH-SAD data at posttreatment: 84 (95.5%) of the 88 patients assigned to CBT-SAD and all of the 89 patients assigned to light therapy.
Table 1 presents baseline demographic characteristics, overall and within each treatment. The study group was predominantly female and white. Roughly one-fourth had comorbid axis I diagnoses or were taking antidepressant medication at baseline.
Table 2 displays current comorbid diagnoses, as ascertained by the original SCID interviewer. Nearly all of the SCID interviews (170 of 177, 96.0%) were archived, with only seven unavailable for verification. The independent rater corroborated the interviewer’s diagnosis of major depressive disorder, recurrent, with seasonal pattern for all 170 available SCIDs and in most cases agreed with the interviewer on the presence or absence of axis I comorbidity (156 of 170, 91.8% agreement; kappa=0.79), presence or absence of any anxiety disorder (150 of 170, 88.2%; kappa=0.76), and number of comorbid diagnoses (144 of 170, 84.7%; kappa=0.66). Diagnostic agreement was lower at the level of specific diagnoses: the kappa values were 0.64 for specific phobia, 0.73 for social anxiety disorder, 0.77 for generalized anxiety disorder, and 0.74 for panic disorder without agoraphobia.
Treatment Integrity
The random sample of treatment sessions rated for treatment integrity included 48 CBT-SAD sessions, balanced across 16 groups, three therapists, and 12 sessions (four of each session), and 16 of the 61 light therapy instructional sessions. The interrater reliability intraclass correlation coefficient (ICC) was 0.76 for the modified Collaborative Study Psychotherapy Rating Scale. The scale contains separate cognitive-behavioral and clinical management scales, and for them the ICC values were 0.96 and 1.0, respectively. Mann-Whitney tests compared CBT-SAD versus light therapy on the cognitive-behavioral and clinical management scales and their subscales to measure success at discriminating treatment content. When the mean score of the two raters for each session rated was used, CBT-SAD and light therapy were distinct from one another on the cognitive-behavioral scale and all eight of its subscales and on the clinical management scale and all four of its subscales (p<0.001 in all cases except the relapse prevention subscale [p=0.05] of the cognitive-behavioral scale). Inspection of the mean ratings on the subscales of the cognitive-behavioral scale, averaged across the two raters and both CBT-SAD sessions within a given treatment week, suggested adherence to CBT-SAD manual content with psychoeducation covered earliest, followed by behavioral activation, cognitive therapy, and relapse prevention.
Light Therapy Prescriptions
Most light therapy participants (83 of 89) required at least one adjustment to the light therapy prescription. Of these 89 patients, 69 were prescribed morning-only light therapy and 20 were prescribed combined morning and evening light therapy. At week 6, the dosage of morning light therapy was 15 minutes for two participants, 20 minutes for one, 30 minutes for 14, 45 minutes for 28, 50 minutes for one, 60 minutes for 32, 75 minutes for 10, and 90 minutes for one. The dosages for morning plus evening light therapy were 20+10 minutes for one participant, 30+10 minutes for one, 30+15 minutes for one, 30+30 minutes for one, 30+45 minutes for one, 45+15 minutes for eight, 60+15 minutes for five, and 60+30 minutes for two. Two subjects could not tolerate our minimum prescription of 30 minutes total of light therapy per day and ended treatment at 15-minute morning-only light therapy. One reported headaches during each week that 30 minutes was prescribed. The other reported overactivity, which was determined not to be hypomania according to an outside psychiatry consultation. The subjects for whom morning-only light therapy was prescribed did not differ from those with prescriptions of morning plus evening light therapy on posttreatment depression severity as measured with the SIGH-SAD (mean scores of 11.5 and 11.3, respectively; p=0.91) or the BDI-II (mean scores, 7.1 versus 7.2; p=0.96).
CBT-SAD Session Attendance
On average, participants assigned to CBT-SAD attended 9.1 sessions (SD=3.5). The majority of those who withdrew (seven of 13) did not attend any sessions (for the other six the range was 2–7 sessions). Of the 88 participants in this condition, seven attended no sessions, one attended two, two attended four, one attended five, four attended six, six attended seven, seven attended eight, four attended nine, 11 attended 10, 23 attended 11, and 22 attended all 12 sessions.
Continuous Treatment Outcomes (Depression Severity)
Table 3 displays estimated mean SIGH-SAD and BDI-II scores and the number of participants who provided data at each time point within each treatment. As expected, mixed-effects regression models of SIGH-SAD scores revealed a significant main effect of time (F=170.76, df=6, 920, p<0.0001) and a nonsignificant interaction of treatment and time (F=0.62, df=6, 920, p=0.72). The 95% confidence interval (CI) for the observed difference (CBT-SAD minus light therapy) of 1.5 points in posttreatment SIGH-SAD scores was –0.6 to 3.6. Across treatments, the estimated mean SIGH-SAD score was 27.8 (SD=5.5) at pretreatment; at weeks 1–5 the mean scores were 23.3 (SD=7.9), 19.9 (SD=7.8), 18.0 (SD=7.4), 15.1 (SD=7.6), and 13.9 (SD=6.9); and at posttreatment the mean was 12.2 (SD=6.8). The SIGH-SAD score at each time point differed significantly from all others (p<0.01) except that the difference between the scores at weeks 4 and 5 fell short of significance (p=0.07). The same pattern was observed on the HAM-D and the subscale for atypical symptoms, and the effect of time was significant for both the HAM-D (F=119.80, df=6, 920, p<0.001) and atypical symptom subscale (F=102.31, df=6, 920, p<0.001).
On the BDI-II, regression models revealed a significant main effect of time (F=335.53, df=2, 332, p<0.0001) and a nonsignificant interaction between treatment and time (F=2.11, df=2, 332, p=0.13). The 95% CI for the observed 1.0-point difference between CBT-SAD and light therapy on the posttreatment BDI-II score was –1.3 to 3.3. The estimated mean BDI-II scores were 23.05 (SD=8.9) at pretreatment, 12.97 (SD=7.0) at midtreatment, and 7.66 (SD=6.4) at posttreatment. The BDI-II score at each time point differed from all of the others at p<0.0001.
Treatment Moderators
Of potential moderators of treatment effects, only baseline comorbid diagnostic status was associated with depression severity throughout treatment. Across all time points, participants with a comorbid diagnosis had significantly higher SIGH-SAD scores than those without (F=4.83, df=1, 175, p=0.03). This effect was driven primarily by atypical depression severity; the main effect of comorbidity was significant for the atypical symptom subscale (F=5.57, df=1, 175, p=0.02) but not for the HAM-D (F= 2.11, df=1, 175, p=0.15). Similarly, participants with comorbidity had significantly higher BDI-II scores (by about 2 points at each time point) than those without comorbidity (F=5.67, df=1, 175, p=0.02). There were no significant two-way interactions between comorbidity and time and no significant three-way interactions of comorbidity, treatment, and time.
Dichotomous Treatment Outcomes (Remission Status)
Table 4 displays the proportions of remissions in each treatment group according to SIGH-SAD and BDI-II criteria with corresponding statistics, including the 95% CI for the difference between CBT-SAD and light therapy in remissions. CBT-SAD and light therapy did not differ in proportions of remissions according to either outcome measure. For SIGH-SAD remission, using either a worst-case scenario for CBT-SAD (considering the four missing patients in the CBT-SAD group as not in remission; p=0.82) or a best-case scenario (remission for all four missing patients; p=0.71) did not alter the conclusions. Similarly, for BDI-II remission, neither the worst-case scenario for CBT-SAD (nonremission for the four missing patients in the CBT-SAD group and remission for the one missing patient in the light therapy group; p=0.16) nor the best-case scenario (remission for the four missing patients and nonremission for the one missing patient in the light therapy group; p=0.50) resulted in a significant difference between treatments.
Effects of Therapist and CBT-SAD Group Membership
In variance component analysis (i.e., with therapists and CBT-SAD groups considered as random effects), the proportion of variance attributable to therapist (ICC) was zero for posttreatment SIGH-SAD score, either with or without adjustment for baseline score. For posttreatment BDI-II score, the ICC for therapist was zero when not adjusted and 0.009 when adjusted for baseline score. The ICCs for CBT-SAD group membership were as follows (without and with adjustment for baseline score, respectively): 0.045 and 0.089 on the SIGH-SAD and 0.044 and 0.031 on the BDI-II at posttreatment. When we analyzed therapist as a fixed effect, controlling for CBT-SAD group as a random effect and baseline score as a fixed effect, there was still no significant difference between therapists on either the SIGH-SAD (p=0.61 for differences among the three therapists; p=0.97 for the principal investigator versus the other two) or the BDI-II (p=0.66 for differences among the three therapists; p=0.55 for the principal investigator versus the other two).
Discussion
This randomized clinical trial is, to our knowledge, the largest comparison of the effectiveness of CBT-SAD and light therapy in the treatment of SAD. This trial was based in Burlington, Vermont (44.5° N), where the mean daily photoperiod (i.e., hours:minutes from sunrise to sunset) is only 9:36 in November, 8:54 in December, 9:17 in January, and 10:27 in February.
Depression severity improved significantly over 6 weeks of treatment with no significant differences between CBT-SAD and light therapy. Additionally, remission status did not statistically differ between treatments. The pattern of results was consistent across two measures, symptoms on the SIGH-SAD assessed by a blind interviewer and patient-rated symptoms on the BDI-II. The observed differences between treatments in posttreatment depression scores (differences of 1.5 on the SIGH-SAD and 1.0 on the BDI-II) and in the proportions in remission (differences of 0.004 and 0.076 according to the remission definitions based on the SIGH-SAD and BDI-II, respectively) were small and not clinically meaningful. Baseline characteristics including sex, age, race, pretreatment depression severity, comorbidity status, and antidepressant medication status did not predict differential outcome in CBT-SAD versus light therapy. However, participants who entered the trial with a comorbid diagnosis remained more depressed across the 6 weeks relative to those without comorbidities.
The outcomes for CBT-SAD and light therapy virtually replicate those from our earlier study (
6) (mean posttreatment SIGH-SAD scores of 12.7 with CBT-SAD and 12.9 with light therapy), which included a wait list as a control condition (mean posttreatment SIGH-SAD score of 23.1). The current findings extend our prior work by demonstrating that the CBT-SAD outcomes of the patients assigned to the two community therapists were comparable to those of patients assigned to the principal investigator. This result increases confidence that the results seen with CBT-SAD are not specific to the principal investigator, who was the developer of CBT-SAD and the sole interventionist in prior trials. This study also showed that our prior short-term results for CBT-SAD and light therapy generalize to a more ecologically valid SAD sample, including patients with baseline comorbidities and stable antidepressant medications.
Limitations include the single site with a principal investigator known for CBT-SAD and a racially homogeneous sample. However, our prior studies were conducted in the greater Washington, D.C., metropolitan area, included a more diverse sample, and yielded similar results (
6), suggesting that the previous findings generalize to Vermont residents. CBT-SAD and light therapy inherently differ in delivery format (in a group twice weekly versus individually daily at home). Attrition in light therapy was surprisingly low, whereas the 15% attrition in CBT-SAD is consistent with attrition in trials of cognitive therapy for nonseasonal depression. It is noteworthy that about half (7/13) of patients who dropped out of CBT-SAD were unwilling to start the treatment.
The current study represents the first wave in a longer-term project, in which subjects were followed for 2 years after acute-episode treatment to examine outcomes one and two winters later. The primary outcome in the broader study is depression recurrence after treating the index episode with CBT-SAD or light therapy, which is yet to be reported. In our preliminary study, CBT-SAD was superior to light therapy in terms of recurrences and symptom severity the next winter (
16).
In conclusion, these findings suggest that CBT-SAD and light therapy are comparably effective treatment modalities for targeting acute SAD. Accordingly, CBT-SAD should be disseminated into practice and considered as a viable alternative to light therapy in treatment decision making.
Acknowledgments
The authors thank Lorinda Michael Roberts, M.A., for assistance with project administration; Arnold I. Kozak, Ph.D., and Teresa Linares Scott, Ph.D., for serving as study therapists; David A.F. Haaga, Ph.D., for providing consultation on the project’s implementation; and James L. Jacobson, M.D., for serving as medical monitor.