Diverse lines of evidence from genetic, epidemiological, and biomarker studies suggest that immune- and inflammation-related abnormalities play an important role in the disease process of schizophrenia. For example, multiple genome-wide association studies have identified variants in genes involved in immune and inflammatory signaling pathways that associate with schizophrenia (
1–
6). In addition, exposure to infectious diseases in pregnancy (
7,
8), including evidence of maternal response to infection such as higher serum levels of proinflammatory cytokines (
9) and inflammatory biomarkers (
10), have been linked to higher rates of schizophrenia in offspring (
11). Furthermore, higher rates of autoimmune illnesses (
12,
13) and higher levels of proinflammatory cytokines, such as interleukin 6 (IL-6) in the serum (
14–
16) and in the prefrontal cortex (
17,
18), have been reported in subjects with schizophrenia.
Consistent with these findings, we and others have reported evidence of immune activation in the prefrontal cortex in schizophrenia, including higher mRNA levels for the viral restriction factor interferon-induced transmembrane protein (IFITM), which inhibits the processes involved in viral entry and replication (
19–
21). Schizophrenia subjects with higher IFITM mRNA levels also had greater disturbances in markers of prefrontal cortex GABA neurons, suggesting that altered immune function may be involved in cortical circuitry alterations in the disorder. These findings suggest that investigating the pathogenesis of IFITM overexpression may provide a useful window into the role of altered prefrontal cortex immune markers in the pathophysiology of schizophrenia. However, the upstream factors that contribute to elevated mRNA levels for IFITM and other immune-related markers (
17,
18) in the prefrontal cortex in schizophrenia are not known. For example, higher IFITM mRNA levels may be attributable to immune activation (
18); however, it is not known whether higher levels of the cytokines and transcriptional regulators that regulate the expression of IFITM (
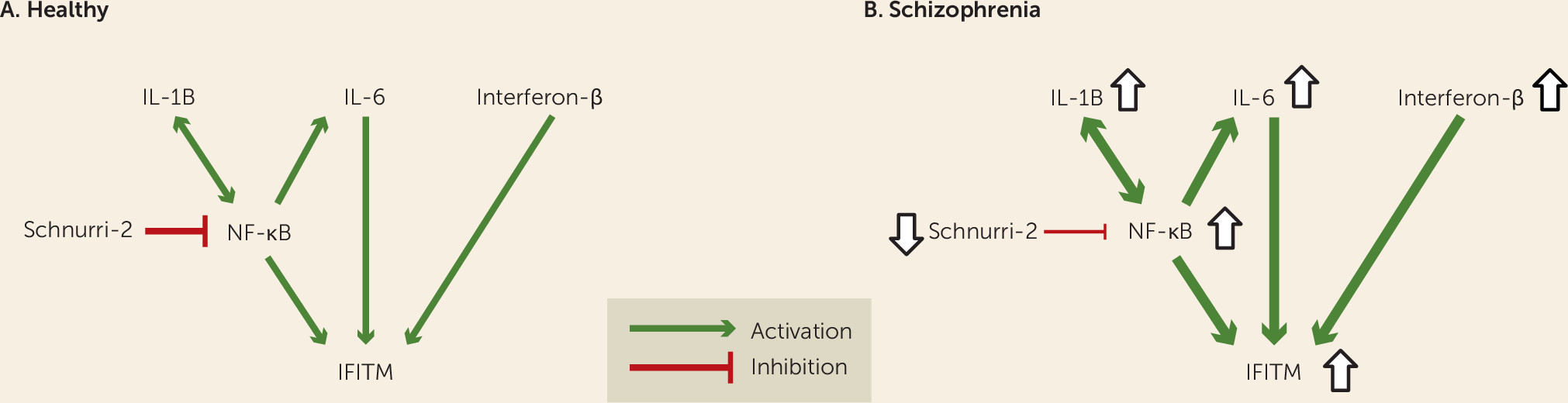
Figure 1A) (
22–
24) and other immune-related markers are present in the prefrontal cortex of the same schizophrenia subjects who have higher IFITM mRNA levels (
21). Furthermore, such correlative evidence of a relationship between IFITM and cytokine levels in the prefrontal cortex, if present in schizophrenia, would require additional proof-of-principle testing of cause and effect using animal models of immune stimulation.
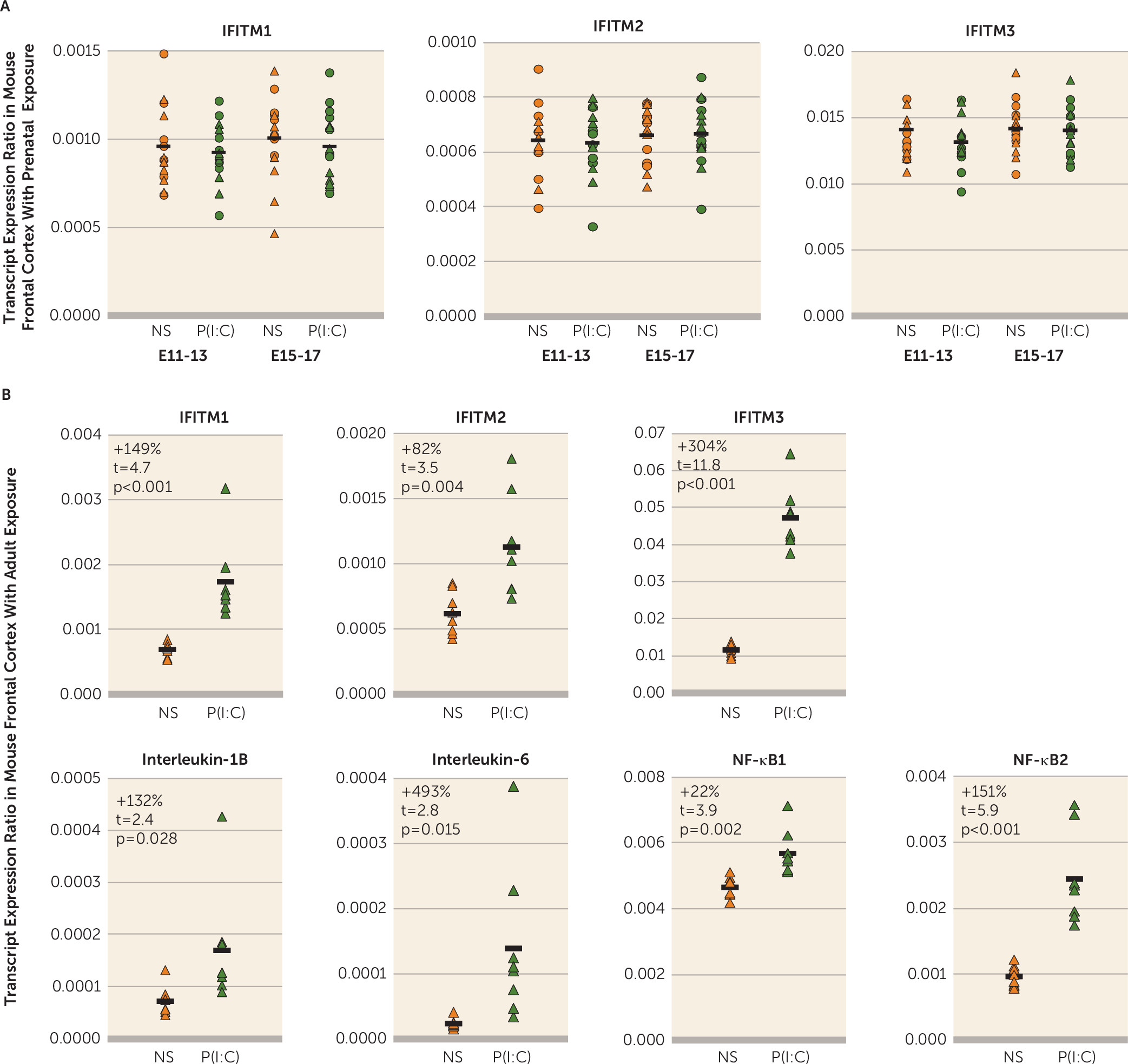
It is also unknown whether higher IFITM mRNA levels in the prefrontal cortex in schizophrenia reflect a long-lasting maladaptive response to an insult that occurred much earlier, such as maternal immune activation in utero, as suggested by epidemiological findings (
7–
11). This hypothesis is supported by experimental findings that maternal immune activation using the immune stimulant poly(I:C) in mice leads to higher cytokine levels in fetal brain (
25) that may persist to a varying extent postnatally (
26). In addition, maternal immune activation has also been reported to produce epigenetic modifications to the promoter regions of genes that can alter gene expression postnatally (
27).
Discussion
In this study, we sought to determine whether higher IFITM mRNA levels and other immune-related disturbances in the prefrontal cortex of schizophrenia subjects are more consistent with being attributable to 1) the consequence of an ongoing molecular cascade contributing to immune activation or 2) a long-lasting maladaptive response to an immune-related insult that occurred during prenatal development. In the prefrontal cortex of the same schizophrenia subjects that we previously reported to have higher IFITM mRNA levels (
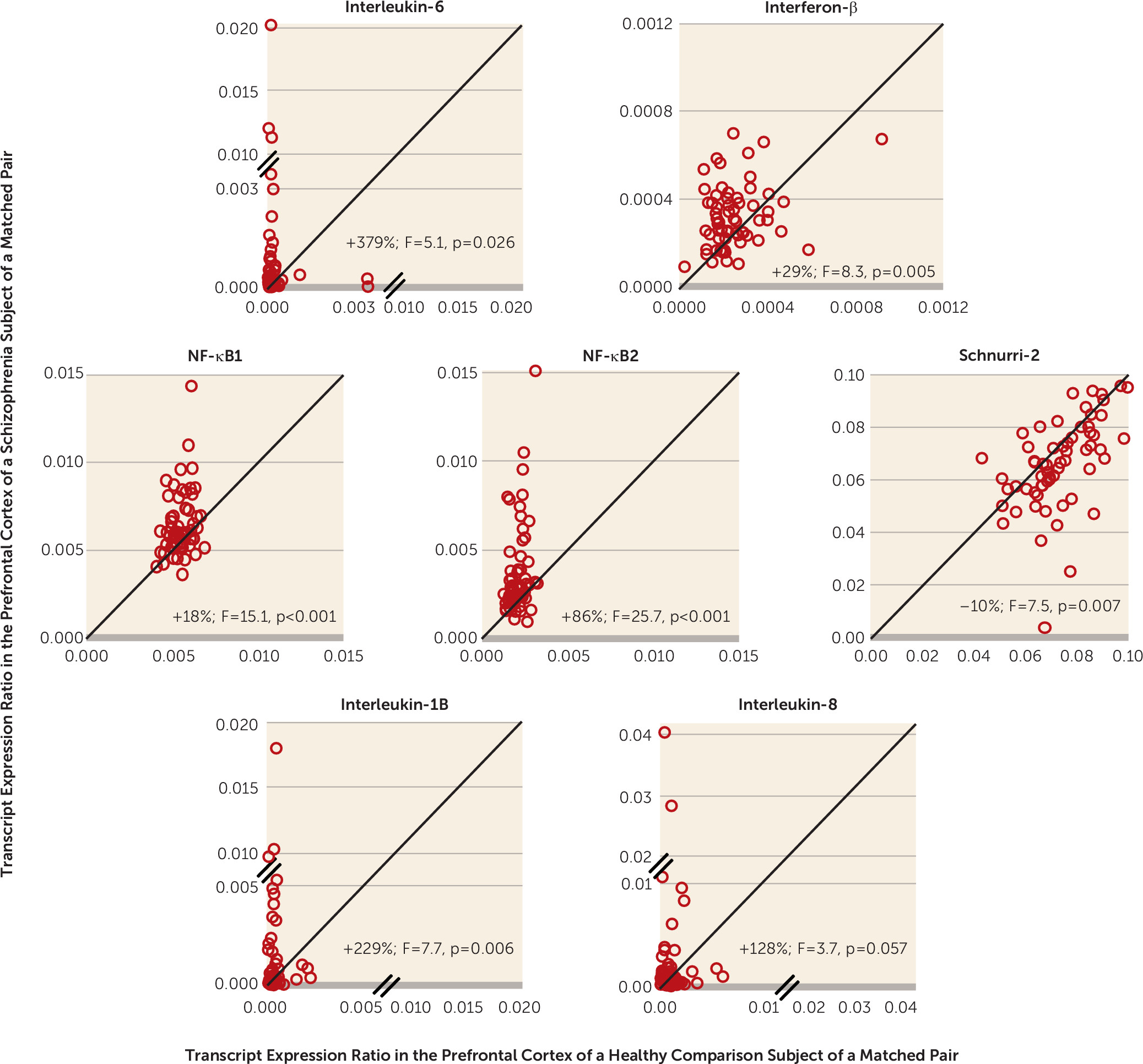
21), we found markedly higher mRNA levels for cytokines (e.g., IL-6 and interferon-β) and transcriptional regulators (i.e., NF-κB) that induce IFITM expression, as well as lower mRNA levels for an NF-κB site-binding protein (i.e., Schnurri-2) that inhibits IFITM expression (
Figure 1B). Furthermore, the within-subject correlations of IFITM mRNA with cytokine and transcription factor mRNA levels in the prefrontal cortex support the prediction that the latter could be causal of the former. This idea was further strengthened by proof-of-principle evidence that exposure to immune stimulation in adult mice produces both higher cytokine levels and higher IFITM levels in the frontal cortex in a manner similar to that seen in schizophrenia. In contrast, exposure to immune stimulation in utero (i.e., maternal immune activation) did not recapitulate schizophrenia-related immune marker expression abnormalities in the frontal cortex.
In the prefrontal cortex of schizophrenia subjects, the markedly higher mRNA levels for cytokines and transcription factors that induce IFITM expression (e.g., IL-6, interferon-β, and NF-κB), and lower mRNA levels for Schnurri-2, which suppresses IFITM expression, suggest that this combination of molecular mechanisms accounts for the elevated levels of IFITM mRNAs in the illness (
Figure 1B). In addition, mRNA levels of NF-κB, a critical transcription factor that regulates the expression of many immune-related genes, including the cytokines IL-1B, IL-6, and IL-8 (
41,
42), were higher in schizophrenia. Furthermore, mRNA levels for these cytokines and transcriptional regulators were significantly correlated with IFITM mRNA levels. These findings support the idea of a complex cascade of immune activation in the prefrontal cortex in schizophrenia (
Figure 1B). Consistent with this interpretation, Fillman et al. also recently reported (
18) higher mRNA levels for IL-6 and IL-8 in the prefrontal cortex in schizophrenia subjects. Furthermore, higher densities of activated microglia, which produce cytokines in the brain, have been reported in the prefrontal cortex in schizophrenia (
18,
44,
45). Similarly, in vivo positron emission tomography studies have also found higher [
11C]-PK11195 binding, a measure of activated microglia, in the hippocampus and cerebral cortex in schizophrenia (
46,
47). Taken together, these findings provide a striking convergence of evidence for a molecular cascade of immune activation in the prefrontal cortex in schizophrenia and suggest that this molecular cascade could be further explored in studies that directly quantify immune marker protein levels in the prefrontal cortex and directly determine the cell types that overexpress these immune markers in the disorder.
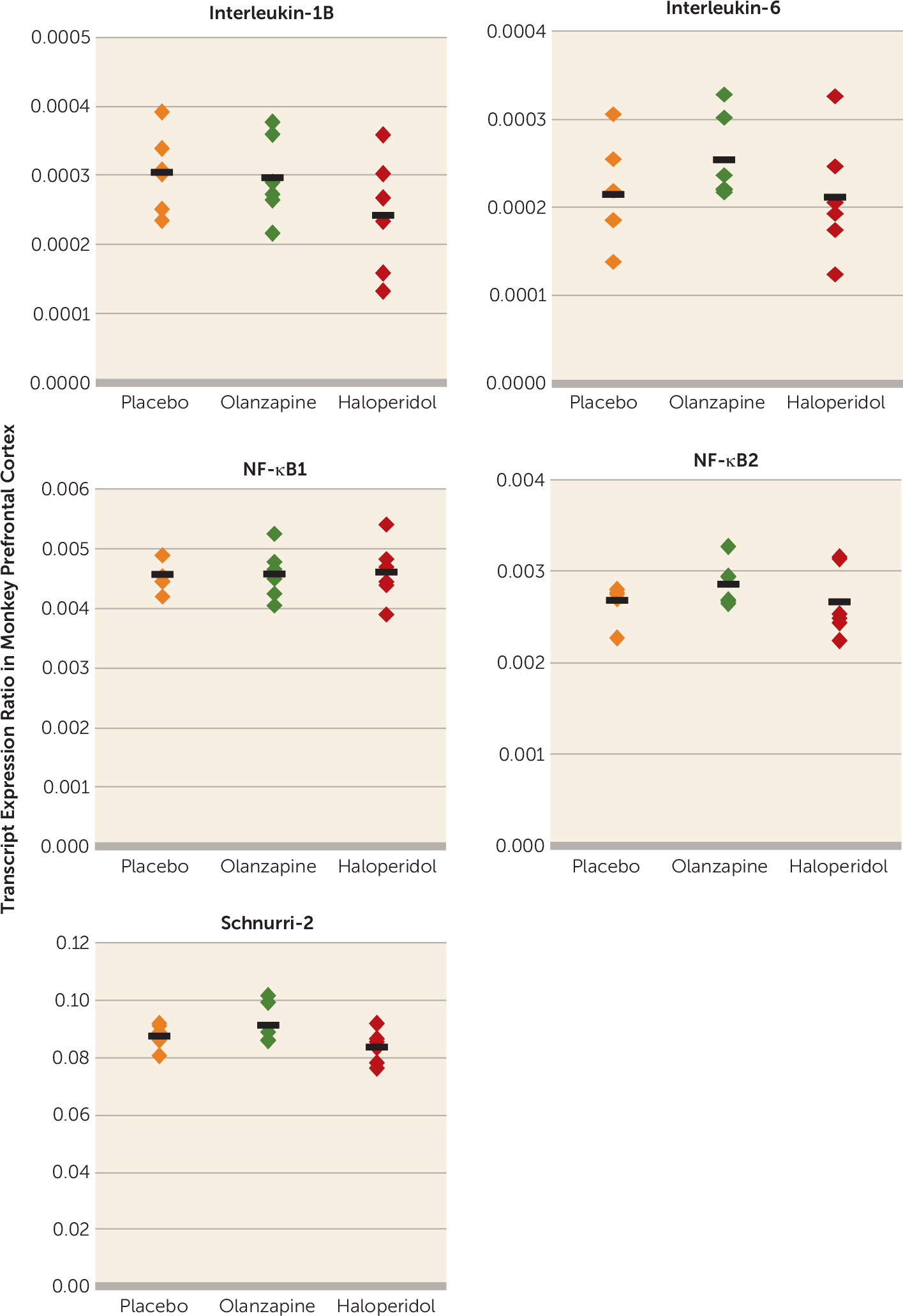
It is also possible that our findings reflect the consequences of schizophrenia that can be accompanied by life conditions (e.g., chronic institutionalization) that may carry an increased risk of exposure to infectious diseases. Although we cannot definitely exclude that possibility, several lines of evidence suggest that our findings do reflect the neurobiology of schizophrenia. Our subjects come from a community-based population (i.e., individuals with unexpected deaths and subsequent autopsies) and were not chronically institutionalized, and altered immune-related marker mRNA levels in schizophrenia did not appear to be attributable to potential confounders such as the presence of immune/inflammation-related illness or use of NSAIDs, psychotropic medications, or tobacco at time of death.
Several epidemiological studies suggest that exposure to maternal immune activation is a risk factor for schizophrenia in offspring (
7–
11). Furthermore, maternal immune activation has also been reported to produce epigenetic modifications that can alter gene expression postnatally (
27). We therefore tested the hypothesis that maternal immune activation may contribute to higher mRNA levels for IFITM and other immune markers in the prefrontal cortex in schizophrenia. However, we found that several days of maternal immune activation in middle or late gestation in mice did not lead to a persistent elevation in mRNA levels for IFITM or several other immune system-related markers in the frontal cortex of young adult offspring such as that seen in schizophrenia. Interestingly, maternal immune activation has been reported to lead to higher cytokine levels in fetal brain (
25), which may still affect the development of neural circuits (
48,
49), even though cytokine mRNA levels are stable in the frontal cortex of young adult offspring with prenatal exposure to immune activation. Furthermore, some evidence suggests that maternal immune activation may interact with genetic risk factors (
50) or adolescent stress (
51) to produce schizophrenia-related abnormalities. These findings suggest that maternal immune activation in isolation may not be a sufficient cause of cortical immune activation in schizophrenia. However, we cannot exclude the possibility that maternal immune activation interacts with other environmental or genetic risk factors that may act in concert to disrupt brain development. In contrast, we found that exposure to immune stimulation in adult mice resulted in a pattern of elevations in cytokine, NF-κB, and IFITM mRNA levels in the frontal cortex similar to that seen in schizophrenia.
Immune activation in the prefrontal cortex may have deleterious effects on cortical circuitry in schizophrenia. For example, we previously reported deficits in GABA neuron-related markers, including the GABA synthesizing enzyme GAD67, the calcium-binding protein parvalbumin, the neuropeptide somatostatin, and the transcription factor Lhx6 in the present cohort of schizophrenia subjects (
30,
33,
52–
55). Furthermore, we previously reported an inverse correlation between IFITM mRNA levels and these GABA neuron-related markers (
21). Fillman et al. similarly found that a subset of individuals with schizophrenia with a “high inflammatory” state had more severe deficits in GABA neuron-related mRNAs, including somatostatin, GAD67, and parvalbumin (
18). In addition, we found lower Schnurri-2 mRNA levels in the prefrontal cortex in schizophrenia, and Schnurri-2 knockout mice exhibit deficits in parvalbumin and GAD67 protein levels (
43). Taken together, these findings suggest that cortical immune activation may have deleterious effects on susceptible components of inhibitory cortical circuitry, including somatostatin and parvalbumin neurons. However, additional proof-of-principle studies are needed to determine the nature of the relationship between cortical immune activation and cortical GABA neuron disturbances in schizophrenia. Such studies will help determine whether immune-related markers represent attractive therapeutic targets in the disorder, as supported by preliminary evidence of clinical efficacy of anti-inflammatory agents in the treatment of schizophrenia (
56), in light of our findings that elevated immune markers appear to reflect an active process rather than the remnants of a prenatal scar.