Perinatal depression, defined as depressive illness occurring during pregnancy (antenatal depression) or following childbirth (postpartum depression), affects some 10%–15% of women and confers substantial morbidity, mortality, and personal and societal costs (
1–
4). The clinical presentation of perinatal depression features low mood, anxiety, rumination, and, in severe cases, suicidal or infanticidal ideation (
5). Historically, perinatal depression has been conspicuously understudied compared with major depressive disorder (
6).
Major depressive disorder is defined as marked and persistent depressed mood associated with physical and cognitive signs and symptoms. Depression is common and costly, and it is projected to be the second leading cause of disability worldwide by 2020 (
7–
10). The heritability of major depression has been estimated at 31%−42% (
11,
12). In comparison with other major psychiatric disorders, discerning the genetic basis of depression has proven to be more challenging. Genome-wide linkage studies, candidate gene studies, and genome-wide association studies have not been successful in identifying risk loci that meet contemporary standards for replication (
13). The relatively modest heritability of depression, as compared with other major psychiatric disorders, may be one reason for the substantially lower yield of identified genetic loci (
14). Another reason is that depression is a markedly heterogeneous disorder.
In contrast, perinatal depression may represent a more homogeneous disorder. Perinatal depression occurs in women of reproductive age and is coupled with pregnancy and childbirth. The limited literature on the genetic basis of perinatal depression suggests a heritable component that may be greater than that in major depressive disorder (
15–
17). Two small studies have shown clustering in families (
16,
17). Murphy-Eberenz et al. (
16) reported odds ratios for prediction of sibling status for perinatal depression or postpartum depression between 2.28–3.96. Forty et al. (
17) studied female siblings and found the sibling correlation for postpartum depression to be highest for postpartum depression with an onset within 6–8 weeks following childbirth (tetrachoric correlation coefficient=0.62, 95% CI=0.16–0.88; p=0.01) in women with recurrent depression. An Australian study of 1,676 twins (
15) estimated the heritability of lifetime postpartum depression at 25%.
Given the uncertainty about the degree to which perinatal depression and nonperinatal depression are distinct and the limited literature on the genetic basis of depression during the perinatal period, there is a great need for an improved understanding of the genetic basis of perinatal depression and the extent of the genetic overlap between perinatal and nonperinatal depression. In this study, we used data from a validated screening tool for perinatal depression in 3,427 twins from the Swedish Twin Registry to estimate the relative contributions of genetic (heritability) and environmental risk factors to the liability to perinatal depression in a classical twin design. We then used Swedish population data from over 580,000 sisters and national treatment registers to estimate the heritability of perinatal depression, the heritability of nonperinatal depression, and the genetic and environmental overlap between the two.
Discussion
To our knowledge, this is the largest and most comprehensive genetic epidemiological study of perinatal depression yet reported. Using Swedish national cohorts, we estimated the heritability of perinatal depression with two different approaches. Our classical twin design estimated the univariate heritability of perinatal depression at 54%, and the sibling study estimated it at 44%. Despite the marked difference in observed occurrence of perinatal depression in the two designs (0.6% and 7.6%), the heritability estimates are similar and the confidence intervals overlap, which is consistent with both approaches capturing the same underlying liability for perinatal depression. The different observed occurrence rates are likely explained by the different methodologies—self-report in the twin design and register-based treatment contacts in the sibling design. Thus, the sibling design did not account for women who did not seek treatment for perinatal depression but who would endorse symptoms on a self-report questionnaire (
34).
Division of the perinatal period allowed for estimation of the heritability of antenatal depression at 37% (95% CI=27%−47%) and postnatal depression at 40% (95% CI=31%−49%), with the remaining variance explained by nonshared environment in both disorders (
Table 4). Thus the variance in both antenatal and postnatal depression displayed a pattern similar to the variance in perinatal depression as a whole.
To our knowledge, there has only been one previous heritability study of perinatal depression (
15). This Australian twin study (N=1,676) estimated the heritability of lifetime postnatal depression at 25% (95% CI=13%−42%). Our estimates for lifetime perinatal depression at 54% (95% CI=35%−70%) in the twin design and at 44% (95% CI=35%−52%) in the sibling design indicate a larger genetic contribution than in the Australian study.
We estimated the heritability of nonperinatal depression at 32%, which is slightly lower than previous estimates of major depressive disorder (
12). However, we included only parous women, and we separated the perinatal and nonperinatal depressive episodes.
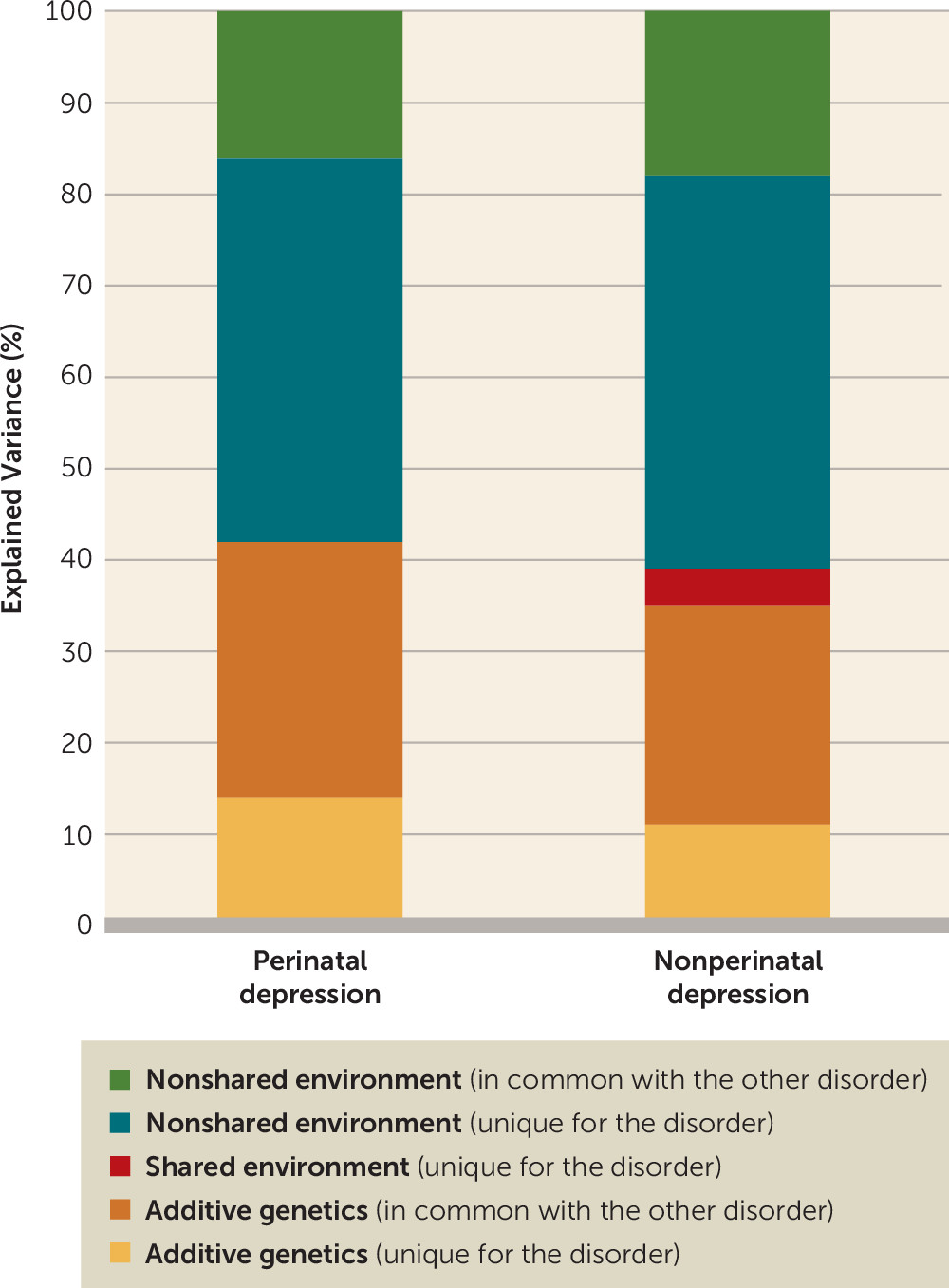
In our bivariate heritability analysis of perinatal depression and nonperinatal depression, 14% of the variance in perinatal depression was explained by genetic factors unique for perinatal depression and 28% by genetic factors shared with nonperinatal depression. In other words, of the total genetic variation for perinatal depression, two-thirds is shared with nonperinatal depression and one-third is unique for perinatal depression.
The heritability estimates of perinatal depression in these two analyses may have particularly important implications. A critical issue facing genetic research in unipolar mood disorders is etiological heterogeneity. It is likely that there are multiple “types” of persistent depressive disorders. Considering these disorders as a single entity may effectively combine different sets of genetic and environmental etiological processes, resulting in higher prevalence and lower heritability (
35–
37). This combination is arguably unfavorable for genomic discovery efforts (
38).
Thus, we hypothesize that perinatal depression represents a form of unipolar mood disorder that could be prioritized for genomic discovery efforts. In effect, we suggest a “divide and conquer” approach to understanding the genomics of unipolar mood disorders. Appropriately powered studies of perinatal depression could deliver genomic findings that are important to disentangling its etiology and that have potential relevance to major depressive disorder. We note that women with perinatal depression are readily ascertained clinically, enabling efficient accrual of large samples. Ultimately, improved identification of women at risk for perinatal depression could lead to targeted interventions to prevent, identify, and more effectively treat perinatal depression in order to minimize adverse sequelae for mother and child.
This study has several strengths. It used both classical twin and sibling designs, and different tools to measure perinatal depression: the validated Edinburgh Postnatal Depression Scale in 3,427 female twins, and treatment contacts from national Swedish registers in over 580,000 sisters, which allowed for separation of depressive illness into perinatal depression and nonperinatal depression, and further division into antenatal and postnatal depression. Additionally, sensitivity analyses suggested that the unique genetic component seen in perinatal depression was not explained by bipolar disorder or schizophrenia (see appendix 1 in the data supplement that accompanies the online edition of this article) and that the unique genetic component was linked to the actual pregnancy (see appendix 2 in the data supplement).
This study also has limitations. The Swedish National Patient Register does not include outpatient admissions before 2001 and it includes no primary care data. The percentage of women being treated for depression exclusively in primary care in Sweden is not known, but the observed occurrence of perinatal depression based on treatment contacts is likely an underestimate of the true prevalence. Furthermore, depression identified using the National Patient Register will likely be on the more severe end of the spectrum compared with depression identified using the Edinburgh Postnatal Depression Scale. If the assumption of an underlying continuous liability in the threshold model is true, this should not affect the heritability estimates. Indeed, increasing or decreasing the Edinburgh Postnatal Depression Scale cutoff to capture more or less severe depressive illness did not change the heritability estimates (see appendix 3 in the
data supplement). The observed outcome occurrence of perinatal depression among the twins using the Edinburgh Postnatal Depression Scale was 7.6%, which is lower than observed in other studies (10%−15%) (
1–
4). This could be due to the participants being exclusively twins and to use of retrospective assessments rather maternal health care visit assessments. When estimating the heritability of antenatal and postnatal depression, respectively, the postnatal period had to be expanded to 12 months to include enough cases to allow analysis. While this deviates from the traditional definition of perinatal depression, which covers a 6-month postnatal period, analysis of perinatal depression using a 12-month postnatal period is consistent with a broader definition and revealed almost identical results, with the heritability estimated at 43% (95% CI=34%−51%) and the remaining variance explained by nonshared environment (57%; 95% CI=49%−66%). We were also not able to restrict the postnatal period to the first 4 or 6 weeks. However, early onset of symptoms may not lead to contact with the health care system within this period unless symptoms persist and could therefore remain undetected when using treatment contact data. Examination of differences regarding timing of onset of symptoms in pregnancy versus the postpartum period has been a central issue in recent work (
39), and future research should focus on further elucidating the genetic and biological contributions to the timing of onset of symptoms.
These findings provide important information that will assist clinicians as they counsel their patients regarding the risk and prognosis of perinatal mood disorders. For example, the heritability of a disorder has a direct translational impact in discussions between clinicians and patients. Most patients will ask fundamental questions, such as “Why do I have perinatal depression?,” “Was it my fault?,” and “What is the risk next time?” This study highlights the critical need for clinicians providing obstetrical care to obtain detailed information regarding the patient’s personal and family history of any psychiatric illness that began in a perinatal period. Integration of genetic risk with environmental influences is vital for the appropriate tailoring of individual treatment and discussions of prognosis.
In conclusion, we report the largest heritability studies of perinatal depression to date, including the first bivariate heritability study of perinatal and nonperinatal depression, revealing that one-third of the genetic variance is unique for perinatal depression. We believe that perinatal depression represents a form of unipolar mood disorder that can be utilized by clinicians in discussions with their patients and could be prioritized for genomic discovery efforts.