Major depressive disorder is a leading cause of chronic disability (
1) and mortality (
2) worldwide. Many of the health consequences associated with depression are due to the fact that this disorder predisposes and exacerbates other chronic medical conditions, including diseases related to depression’s vegetative symptoms, such as obesity and diabetes (
3,
4). Importantly, the vegetative symptoms to which these conditions are most closely related, namely appetite and weight changes, are not shared by all patients with depression. Patients with major depressive disorder exhibit marked heterogeneity in appetite, with approximately 48% of adult depressed patients exhibiting depression-related decreases in appetite, while approximately 35% exhibit depression-related increases in appetite (
5). In fact, across large depressed cohorts, appetite and weight changes are often some of the most discriminating symptoms in latent class analyses of depression subtypes (
6–
8). These changes in appetite and weight are 75%−85% stable across depressive episodes (
9,
10), suggesting that they may be trait markers of how depression is manifested within a particular individual.
In light of the variable involvement of eating-related symptoms in depression, it is significant that the orbitofrontal cortex, ventromedial prefrontal cortex, amygdala, insula, and striatal-pallidal neurocircuits are involved in various aspects of appetitive responses to food (
11–
17), and some of these regions also exhibit histopathological and functional differences in major depressive disorder patients that are thought to underlie depression (
18,
19). Remarkably, although increases and decreases in appetite are antipodal criteria in the diagnosis of major depressive disorder, and core regions implicated in the pathophysiology of depression are implicated in appetite, there are no studies comparing the neural responses to food stimuli of patients whose depression manifests with appetite increases versus those for whom depressive episodes result in appetite loss. This is significant because phenotypic variability among depressed patients’ appetitive responses to food may provide a heretofore-unexplored neural phenotype to identify subgroups within depression. In fact, although there is now evidence that functional neuroimaging may classify patients according to how they respond to psychological and pharmacological treatments (
20,
21), there are surprisingly few additional examples of differential neural responses among depression subgroups defined strictly by behavioral symptoms. Evidence of differential neural responses between those for whom depressive episodes are associated with either appetite decreases or increases would support the claim that major depressive disorder is not a unitary disorder, but rather results from distinct pathophysiological constructs. It remains unclear, however, which brain regions might be implicated by such an analysis, as depression-related appetite changes may stem from altered activity within multiple dissociable neural circuits. Functional neuroimaging provides the most direct way to assay these neural circuits and their interactions. In the long term, elucidating which brain regions distinguish between depressed individuals who manifest increased versus decreased appetite may prove seminal to the identification of biomarkers for depression and its subtypes.
We asked healthy adults, unmedicated currently depressed adults reporting depression-associated appetite increases, and unmedicated currently depressed adults reporting depression-associated appetite decreases to perform both a food/nonfood picture-viewing task and a resting-state scan while undergoing functional MRI (fMRI) (see Figure S1 in the data supplement accompanying the online version of this article). In addition, the subjects viewed a different set of food photographs and provided ratings of how pleasant it would be to eat the pictured foods (see Figure S2 in the online data supplement). The study’s methods and analyses were aimed at addressing the following previously unanswered questions. First, do adults who experience depression-related increases and decreases in appetite exhibit differential activity within brain regions that underlie appetitive responses to food stimuli in healthy nondepressed adults? Second, what additional brain regions exhibit differential responses to food stimuli among adults manifesting depression-related increases and decreases in appetite, and what might the anatomical distribution of these differential responses tell us about the pathophysiologies underlying depression subtypes? Third, how does activity in these regions relate to the food pleasantness inferences of depressed subjects with increased and decreased appetite? Fourth, how does the intrinsic functional connectivity of these regions influence these individuals’ food pleasantness ratings?
Method
Participants
Forty-eight right-handed native English-speaking volunteers between the ages of 20 and 50 years with a body mass index (BMI) ≥18.5 participated in the study: 16 participants with major depressive disorder who reported increased appetite in the current depressive episode (female, N=13; age range=24–47 years), 16 participants with major depressive disorder who reported decreased appetite in the current depressive episode (female, N=10; age range=20–50 years), and 16 healthy control subjects (female, N=11; age range=21–48 years). In addition to matching for age, the three groups also did not differ in BMI (see
Table 1) and were in line with the BMI values of the community in which the data were collected (Oklahoma), where nearly 70% of the population is either overweight or obese according to the Centers for Disease Control and Prevention (
http://www.cdc.gov/brfss/brfssprevalence/). Subjects with an unhealthily low BMI <18.5 were excluded from the study. All depressed subjects met DSM-IV criteria for current major depressive disorder. None of the depressed participants had received any psychotropic medication within the preceding 3 weeks (6 weeks for fluoxetine). For additional exclusion criteria and scales used to characterize the sample, see the Methods section in the online
data supplement. After complete description of the study to the subjects, written informed consent was obtained.
Depressed subjects were assigned to either the appetite increase or decrease groups based on their responses to the questions about appetite changes in the mood disorders module of the Structured Clinical Interview for DSM-IV Axis I Disorders and confirmed in an interview with a research psychiatrist. Anhedonia was assessed using the Snaith-Hamilton Pleasure Scale. Because the Snaith-Hamilton Pleasure Scale rates loss of interest in food and drink more severely than increased interest, we excluded these questions from scoring, and the depression subgroups did not differ significantly in anhedonia severity (
Table 1).
Experimental Design
Subjects were scanned between the hours of noon and 16:00. The timing of scanning relative to the last meal was recorded but not fixed (the mean interval between the last feeding and scanning did not differ across groups; see the Methods section in the online data supplement). Upon entering the scanner, participants underwent an anatomical scan, then an 8-minute resting-state scan during which they were instructed to visually fixate on a small cross in the middle of the display screen and were asked to clear their mind and try not to think about anything in particular. In addition, they performed the food/nonfood picture task. In this task, subjects saw a diverse selection of 180 food photographs and 45 nonfood photographs. Foods included appetizing items high in fat and sugar, as well as fruits and vegetables. Nonfood photographs depicted small, manipulable household and office implements. On the same day, subjects also performed the Food Pleasantness Rating Task, in which they saw photographs of 144 different foods and made ratings of how pleasant it would be to eat each food item at that particular moment. This task requires subjects to make hedonic inferences about the foods. The stimuli depicted four broad classes of food items, including high-fat high-sweet foods (e.g., primarily “dessert” foods such as cake and ice cream), high-fat low-sweet foods (e.g., savory foods such as pizza), low-fat high-sweet foods (e.g., fruits), and low-fat low-sweet foods (e.g., vegetables). For additional details regarding the tasks, stimuli, and stimulus presentation, see the Methods section and Figures S1 and S2 in the data supplement.
MRI Data Acquisition, Image Preprocessing, and Subject-Level Statistical Analyses
Magnetic resonance images were acquired using a General Electric Discovery MR750 whole-body 3-Tesla MRI scanner (GE Healthcare, Little Chalfont, United Kingdom), using a scalable 32-channel digital MRI receiver. The Methods section in the online data supplement provides details of the preprocessing and motion-scrubbing techniques applied to the resting-state fMRI data and of the imaging parameters, data processing, and subject-level regression models applied to the task-based and resting-state imaging data. Briefly, functional data were coregistered to the anatomical scan, slice time corrected, motion corrected, spatially normalized to a standard stereotaxic array, smoothed, and converted to percent signal change of the blood-oxygenation-level-dependent (BOLD) signal. Each subject’s functional imaging data were analyzed using multiple linear regression, with regressors marking the onset of picture stimuli, as well as covariates for motion parameters and signal trends.
Group Analyses
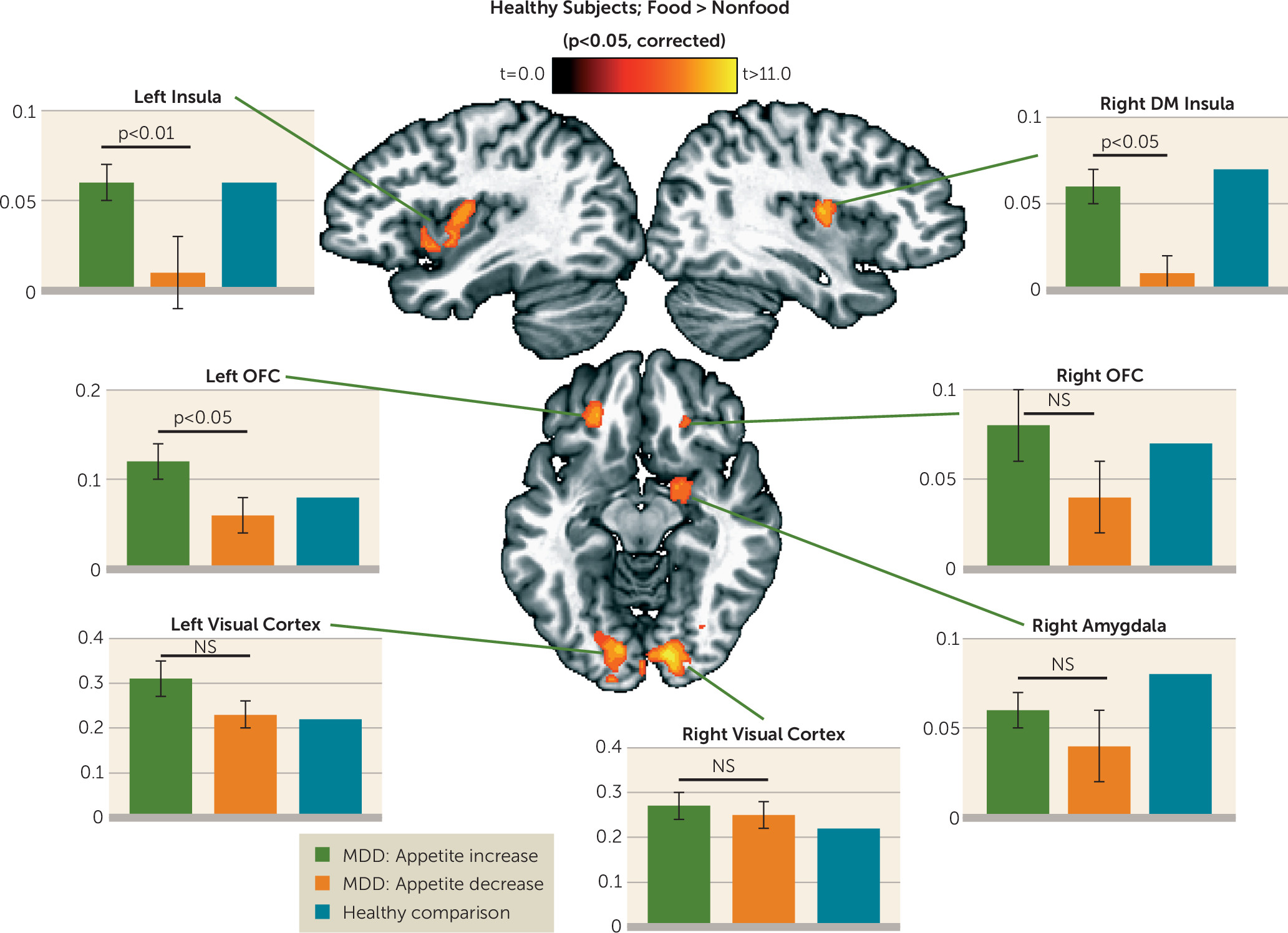
Group analyses of the food/nonfood picture task data were implemented using multiple strategies, all employing two-tailed statistical tests. First, we used a whole-brain voxel-wise random-effects paired-sample t test of the healthy subjects’ beta coefficients for food and nonfood stimuli derived from the subject-level regression analyses. The resulting group statistical map was corrected for multiple comparisons according to procedures described in the Methods section of the online data supplement. We then used random-effects, independent samples t tests on the beta coefficients from the two depressed groups’ subject-level regression analyses within each of seven corrected regions of interest identified in the analyses of the healthy control subjects’ data.
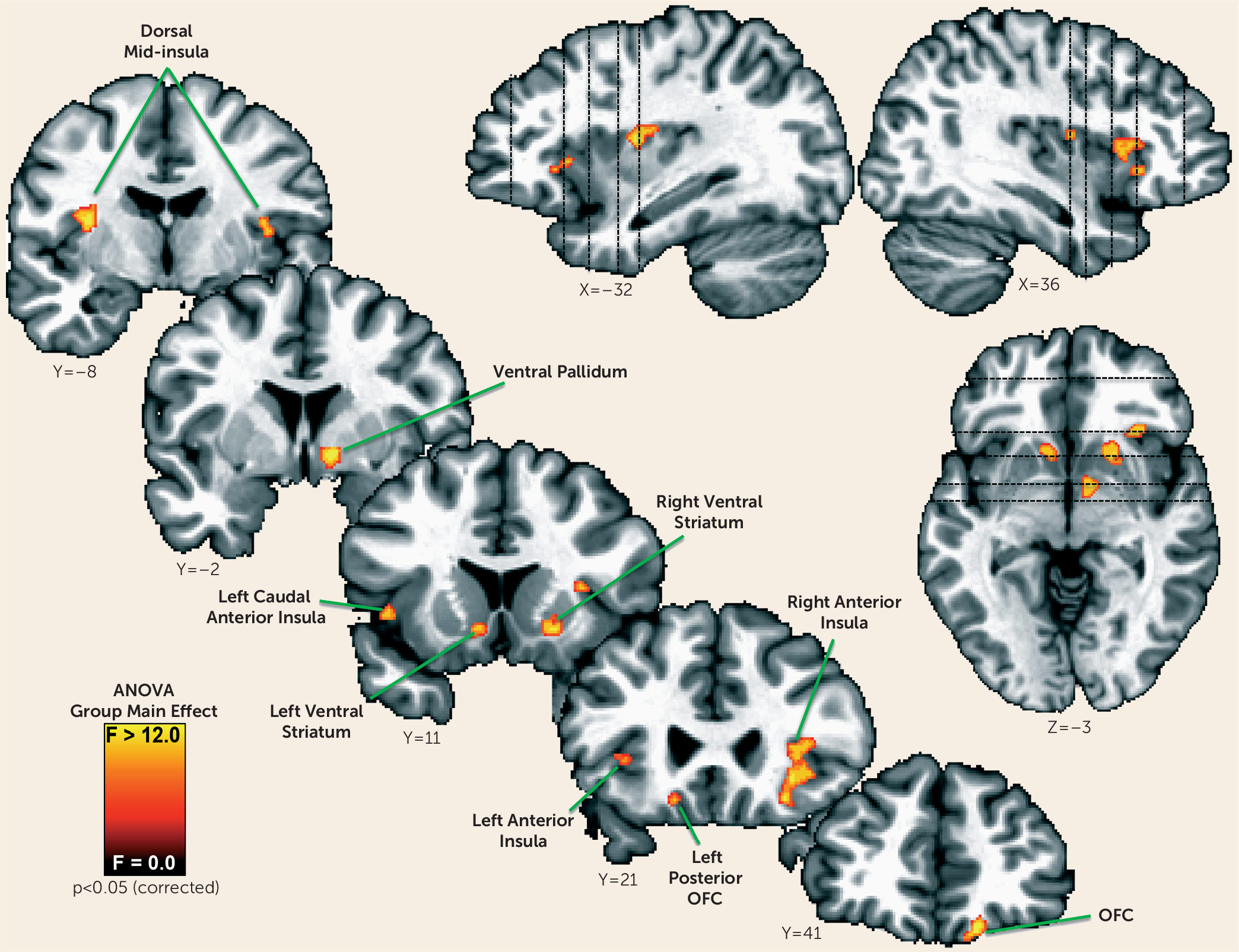
We next determined whether additional regions outside of those normative regions of interest identified in the healthy control subjects might contribute to appetite differences between depressed groups. We therefore performed a voxel-wise one-way analysis of variance (ANOVA) to identify brain regions where the groups exhibited differential responses to food versus nonfood stimuli. The resulting ANOVA F-map was corrected for multiple comparisons using the procedures described in the data supplement. We then conducted follow-up planned comparisons using independent samples t tests of the simple effects within each region of interest to determine which group differences supported the significant ANOVA findings. The post hoc t tests were corrected for multiple comparisons using Tukey’s honest significant difference test.
Between-group t tests were used in the group analyses of the food pleasantness task ratings. Pearson correlations were used to examine the relationship between the subjects’ food pleasantness ratings and activity in the normative regions of interest (described above) that were responsive to food versus nonfood images in the healthy subjects.
To examine the relationship between subjects’ average food pleasantness ratings and the intrinsic functional connectivity of the seven normative food-responsive regions of interest, the resting-state BOLD activity in each region of interest was used as a seed in analyses of the resting-state data, using the AFNI [Analysis of Functional NeuroImages] program 3dttest++, with the subjects’ average pleasantness rating entered as a covariate. The subsequent statistical map was corrected for multiple comparisons (see the data supplement).
Exploratory group analyses examined whether the increased and decreased appetite depressed groups exhibited self-reported differences in sleep characteristics indicative of atypical and melancholic depression. No difference was observed (see the Discussion section and Table S12 in the data supplement).
Discussion
The marked variability in the clinical course and symptomatology of depression suggests that this syndrome can arise from heterogeneous etiologies. This observation has motivated an expanding literature of studies using data-driven analytics to identify depression subtypes from behavioral symptom profiles (for example, see references
7,
8,
24). Nevertheless, there are presently few examples of differential neural responses among subgroups of depressed patients defined a priori by their behavioral symptoms (see references
20,
21). Depression-related increases and decreases in appetite and weight have long been codified as antipodal diagnostic markers in DSM (e.g., DSM-5). Yet while appetite changes have long been recognized as common diagnostic features of depression, and more recently as some of the most discriminating depressive symptoms in latent class analyses of depressive subtypes (
6–
8), there exist no data on differential brain activity among depressed subjects exhibiting increased versus decreased appetites. The present study thus investigated whether depression-related increases and decreases in appetite are associated with differential neural activity in response to food stimuli.
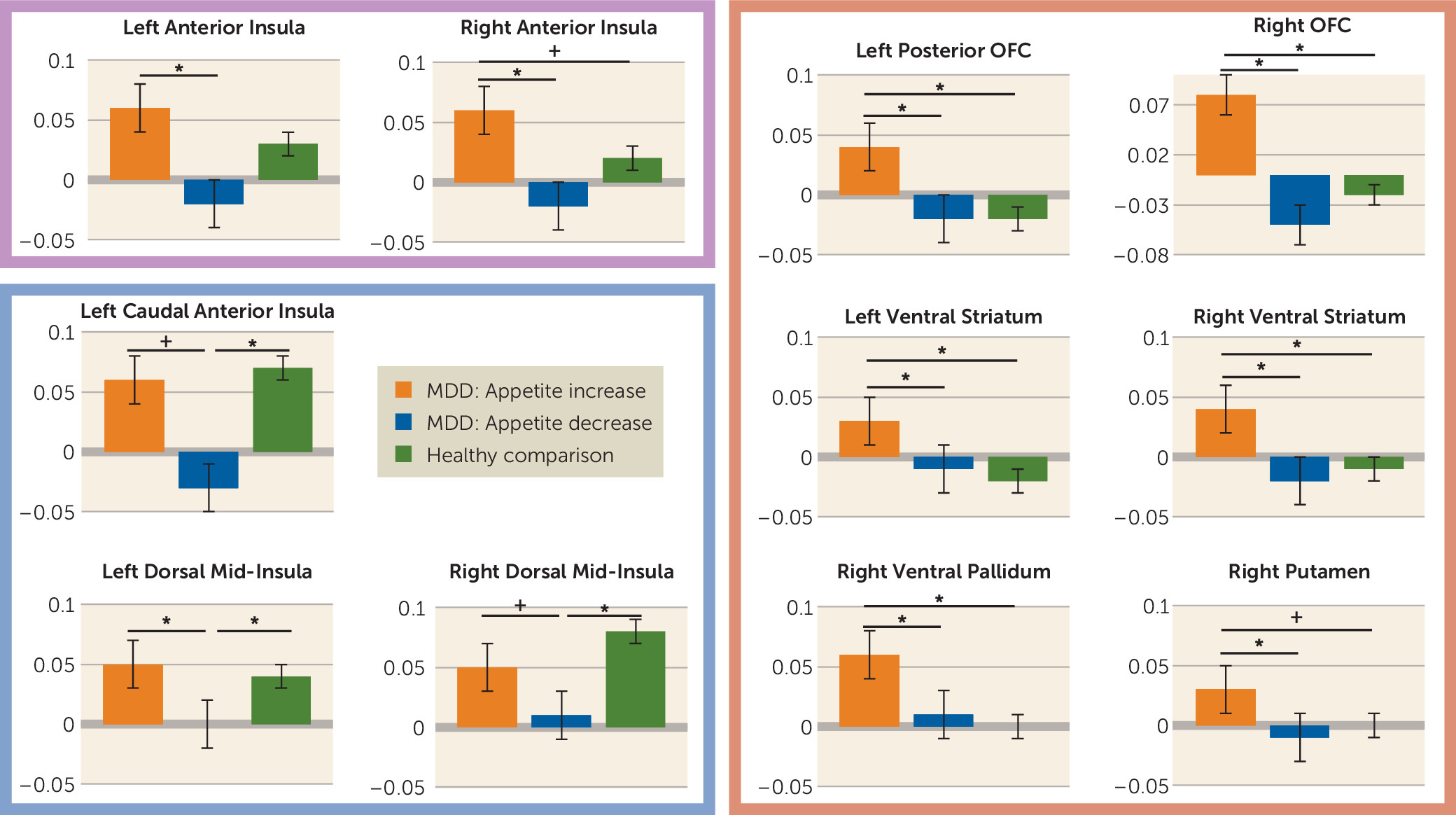
Within regions implicated in neurotypical responses to food stimuli (as defined by food-responsive regions mapped in healthy control subjects), the depressed appetite-increase group exhibited greater responses to food pictures than the depressed appetite-decrease group in the left orbitofrontal cortex and bilateral insula. In other areas, the depressed subjects with increased appetite also exhibited elevated activity compared with both healthy control subjects and depressed subjects with decreased appetite in the ventral striatum, putamen, ventral pallidum, and additional regions of the orbitofrontal cortex. Unexpectedly, we did not observe differences in hemodynamic activity between the depressed appetite-decrease and healthy control subjects in these regions. Rather, the depressed appetite-decrease group exhibited reduced activity relative to the depressed appetite-increase group in the bilateral anterior- and mid-insula. The most prominent effects were located bilaterally in the midinsula, where the depressed appetite-decrease group exhibited significantly less activity than both the depressed appetite-increase and healthy control groups, neither of which differed from each other.
Consistent with our findings of greater activity in the depressed appetite-increase group, the neuroscience literature demonstrates that the orbitofrontal cortex, ventral striatum, and ventral pallidum contribute to various facets of reward processing, including stimulus valuation, motivation, and hedonic experience (
11,
15,
17,
25,
26). Each of these regions has been previously implicated both in appetitive responses to food stimuli and in the pathophysiology of depression. For example, a large human and nonhuman primate research literature demonstrates that the orbitofrontal cortex dynamically encodes the value of stimuli with respect to an individual’s homeostatic needs (for reviews, see references
25,
26). This can be seen both in monkey and human studies of stimulus-specific satiety, where orbitofrontal cortex activity is potentiated for stimuli that are novel and/or meet immediate homeostatic needs but declines as the stimulus is repeatedly presented and the physiological need is reduced (
27). Recent human neuroimaging evidence demonstrates that the anterior orbitofrontal cortex may be particularly sensitive to secondary reinforcers, while the posterior orbitofrontal cortex may be specific to primary reinforcers (
28). In the present study, photographs (secondary reinforcers) of food (a primary reinforcer) elicited hyperactivation in the depressed increased-appetite group in regions corresponding approximately to both BA 11 (anterior orbitofrontal cortex) and BA 13 (posterior orbitofrontal cortex). This suggests that the depressed increased-appetite subjects are excessively responsive to both food cues and food receipt, a possibility that warrants further research. Importantly, the orbitofrontal cortex has also often been implicated in major depressive disorder, since depressed patients exhibit abnormal orbitofrontal cortex volume and blood flow, and orbitofrontal cortex lesions increase the risk of developing depression (
29,
30). These neuroanatomical and functional differences in depression may be associated with histological abnormalities, which have been demonstrated by postmortem neuropathology studies of the orbitofrontal cortex in depressed samples (
31,
32). Likewise, both abnormal reward learning and anhedonia in depression are associated with attenuated activity and dopamine binding in the ventral striatum (
18), a region known to underlie both food motivation (i.e., “wanting”) and hedonic perception (“liking”) (
11). Finally, recent evidence from rodent electrophysiology and human neuroimaging demonstrates that the ventral pallidum is a key component in both the experience and anticipation of food hedonics (
15) and is implicated in depression (
33). (For a discussion of the relationship between findings in the present study and the subjects’ clinical ratings of anhedonia, see the online
data supplement.)
The anterior insula exhibited a pattern in which the depressed group with increased appetite exhibited significantly greater activity to food pictures than the decreased-appetite group, while the hemodynamic response of the healthy group was intermediate between the two depressed groups (
Figure 3). This pattern conceivably may reflect the anterior insula’s role as a center for integrating activity within reward and interoceptive circuitry. This account appears consistent with the anterior insula’s functional connectivity to multiple intrinsic networks in the brain, including reward and interoceptive regions (
34). As such, the pattern of findings in the anterior insula is generally consistent with prior experimental evidence of altered anterior insula activity in depression. For example, both never-depressed adolescents at high familial risk of depression and currently remitted adults with a history of major depressive disorder exhibited weaker activity in the anterior insula and posterior orbitofrontal cortex to the sight and taste of chocolate (
35,
36). Likewise, the anterior insula has emerged as a promising candidate for an imaging biomarker of treatment response in depression, with hypometabolism in this region associated with responsiveness to cognitive-behavioral therapy and hypermetabolism associated with responsiveness to pharmacotherapy using escitalopram (
20,
21). In the present data set, we also find that activity in this region differentiates subgroups of depressed patients defined according to a behavioral phenotype (see the
data supplement for a discussion of these findings in relation to the melancholic and atypical specifiers for major depressive disorder).
In contrast to both healthy control subjects and participants with depression-related increases in appetite, the subjects with depression-related appetite decreases exhibited abnormal activity in the interoceptive cortex. The most pronounced differences were observed bilaterally in the dorsal mid-insula, near the location thought to be the primary gustatory cortex in the human (
37) and where vagal nerve afferent projections from the viscera first synapse in the cortex via connections in the brainstem and thalamus (
38). The mid-insula has been repeatedly shown to play a role in interoception (i.e., a term referring broadly to the perception and integration of autonomic, humoral, and immune signals relating to the homeostatic state). Interestingly, the same region of the dorsal mid-insula observed here also exhibited homeostatically sensitive category-specific responses to food pictures in an earlier study (
16). Specifically, this region exhibited strong responses to food pictures when circulating peripheral glucose levels were low but weak responses when glucose levels were high. This finding implicates the mid-insula both in interoception and in monitoring the body's homeostatic energy needs. These links to the normative function of the dorsal mid-insula, when taken together with the results observed here in depressed subjects with altered appetite, accord well with recent reports demonstrating that depression is associated with both altered interoceptive activity in the dorsal mid-insula and abnormal functional connectivity between this region and other regions implicated in the pathophysiology of depression (e.g., the amygdala) (
39). Because most visceral interoceptive signals reach the brain via the vagus nerve, these findings also appear consistent with evidence for altered vagal function in depression and the efficacy of vagal nerve stimulation for treating major depressive disorder (
40). The accumulating evidence that interoception is compromised in some depressed patients has led to recent theoretical accounts pointing to its role as a central contributor in depression and anxiety (
41,
42). Future research is needed to examine endocrine and peripheral vagal function in depression with appetite loss, as well as to assess these subjects for altered interoceptive processing of homeostatic signals. This interoceptive region is also sensitive to oral somatosensation (
43) and supports overlapping gustatory-interoceptive representations (
44). Additionally, a similar pattern of activity across the groups was observed in the caudal anterior insula, near a region implicated in both taste representation and multimodal olfactory-taste integration (
45,
46).
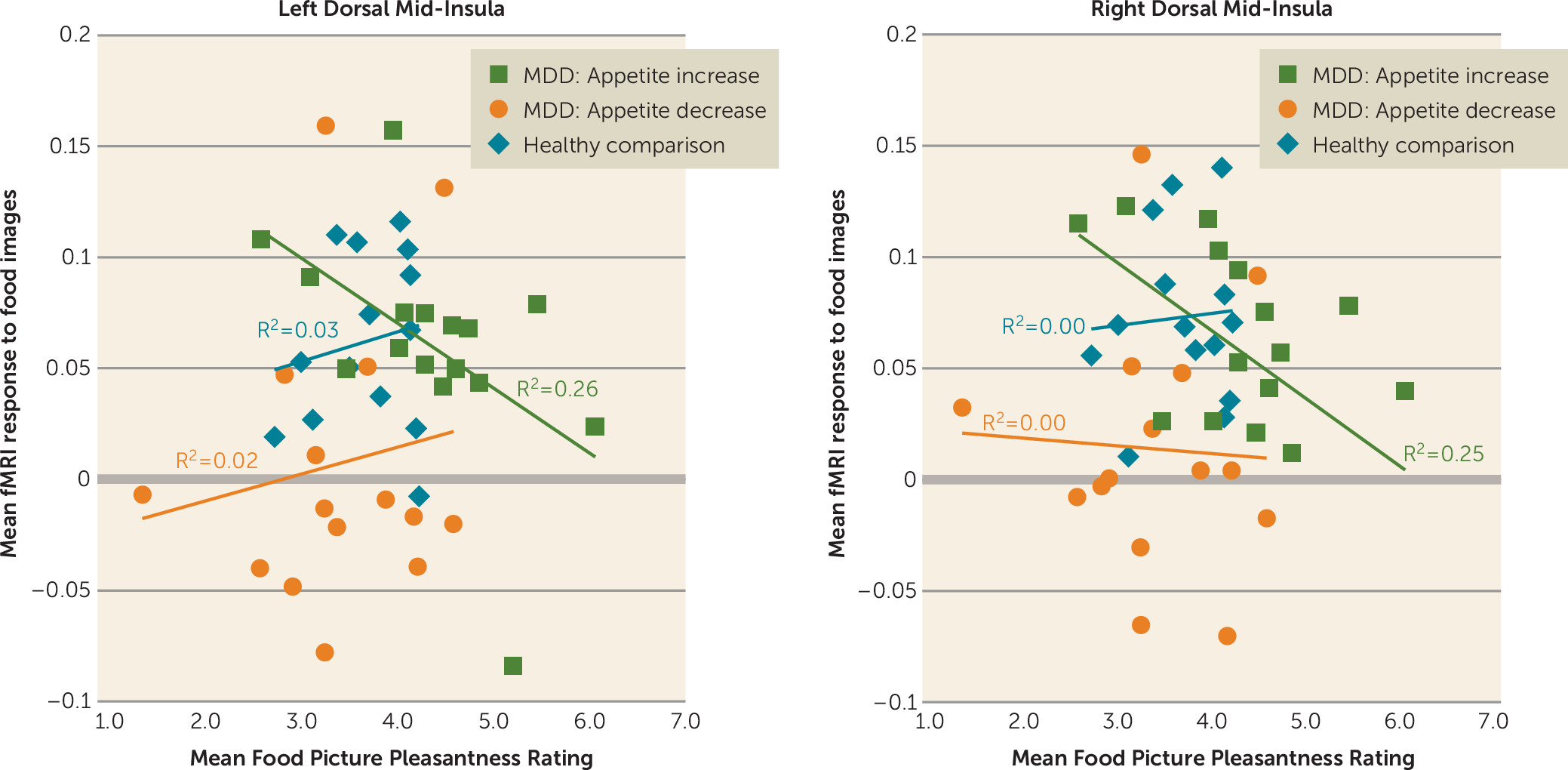
Importantly, activity of the dorsal mid-insula was not implicated only in depression with appetite loss. Compared with the other two groups, the depressed subjects with increased appetite inferred that foods depicted in photographs would be more pleasant to eat, and the activity of both the left and right dorsal mid-insula in response to food images in the depressed increased-appetite subjects was negatively correlated with these food pleasantness ratings (
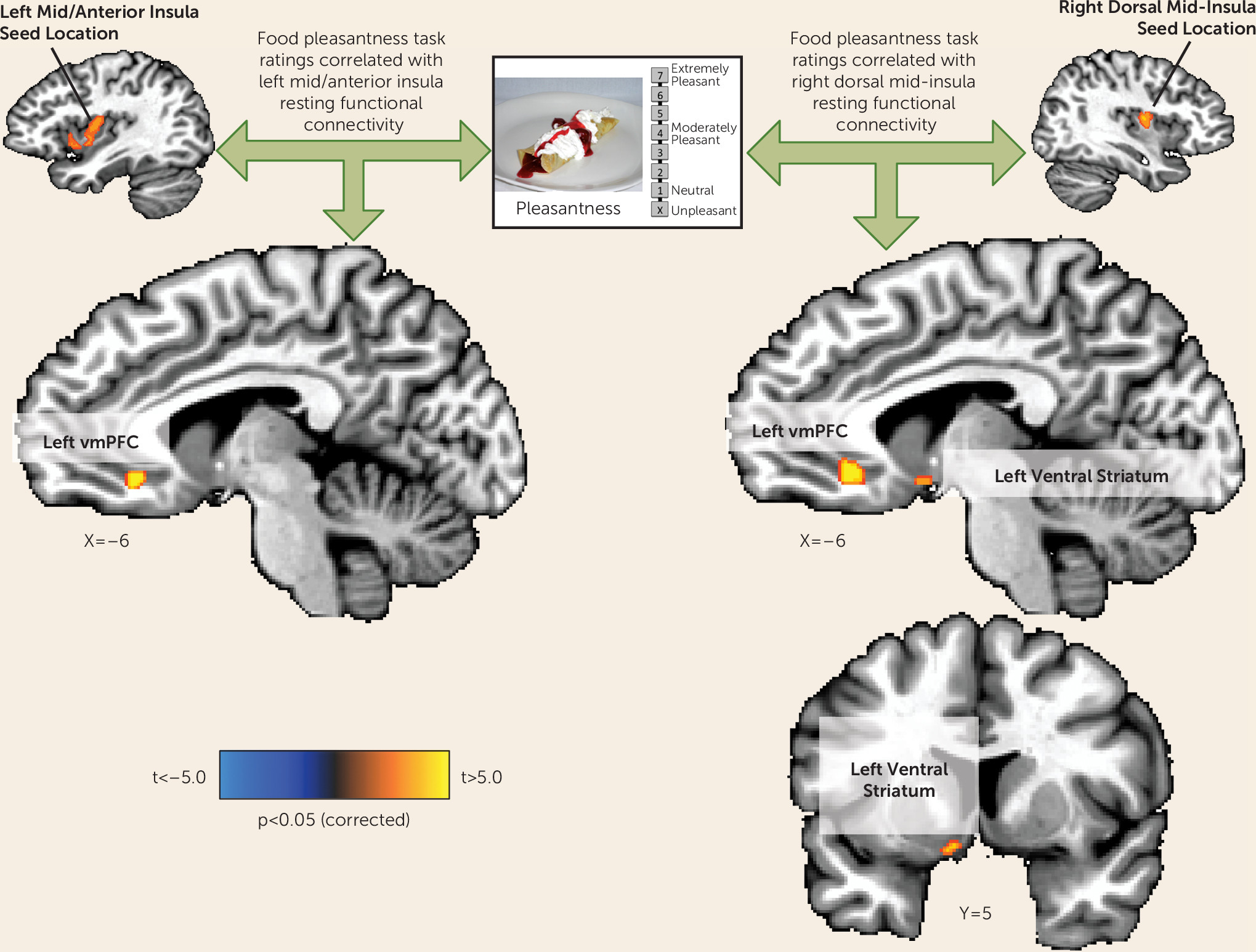
Figure 4). This negative association suggests the interesting possibility that interoceptive signals about the state of the body represented by increased activity of the mid-insula can act as a brake on food anticipation in those with overactive food reward signals (i.e., the depressed increased-appetite group in the present study).
The findings here suggest that if, due to insula pathology, interoceptive representations are aberrant, a depressed individual may not make appropriate interoceptive predictions about the homeostatic consequences of encountered stimuli (e.g., sight or taste of food), resulting in the selection of behaviors that do not maintain homeostatic balance (i.e., either increased or decreased eating). These accounts would thus predict that depression-related appetite loss results from a failure to integrate afferent visceral interoceptive signals about the state of the body with external food cues. Conversely, depression-related appetite increases may result from a dysregulation of the balance between increased reward circuit activity (also observed in the present study) and interoceptive inferences in the insula about the homeostatic consequences of perceived foods.
An implicit assumption in this reward-interoception dysregulation hypothesis is the idea that one or more brain regions integrates both reward and interoceptive information. Based on the findings of both the present study and prior research, the ventral medial prefrontal cortex would seem to be a good candidate. Both the left and right dorsal mid-insula seed regions exhibited functional connectivity to the ventral medial prefrontal cortex that was positively correlated with inferred food pleasantness. Thus, individuals with the strongest functional connectivity between the ventral medial prefrontal cortex and the mid-insula tended to report that foods depicted in photographs would be more pleasant to eat, suggesting that the integrated activity of the two regions influences food judgments. Additionally, the ventral medial prefrontal cortex region observed herein has strong anatomical connectivity to the ventral striatum (
47) (which also exhibited right mid-insula functional connectivity that was correlated with pleasantness ratings) and has been implicated in the integration of hedonic and nonhedonic information in the computation of food value (
48).
Some strengths and limitations of the study design merit comment. Although the sample sizes were relatively low, thereby decreasing the likelihood of detecting less robust group differences, the depressed groups were composed of unmedicated participants, and the depressed subgroups of interest did not differ on BMI, depression severity ratings, or anxiety ratings. Moreover, they did not differ on the Snaith-Hamilton Pleasure Scale once food-related items were removed, indicating that the depressed appetite-increase group was similarly anhedonic to the depressed appetite-decrease group (see the data supplement for results after controlling for depression severity, anxiety severity, and anhedonia). One limitation, however, was that the groups were defined based on self-reported appetite changes, without corroborating evidence of associated weight change. An important next step is to examine whether and how these appetite changes translate into altered eating behavior per se. Relatedly, based on the decreased-appetite depressed group’s average BMI and exclusion of subjects with unhealthily low BMI, these subjects were not malnourished in the sense of excessively low caloric intake, and thus the observed functional changes are unlikely to be accounted for by malnourishment. Nevertheless, in future research it will be important to determine whether appetite changes in depression alter the specific macro- and micronutrient content of depressed individuals’ diets. It will also be important in future studies to attempt to recruit moderately/severely depressed subjects who do not exhibit appetite changes and examine their neural response to food cues, relative to the other three groups described in the present study. Finally, it may also be important in future studies to determine whether the activations observed in gustatory/interoceptive insula cortex reported here might result from autonomic changes or gustatory recall associated with seeing the food stimuli.
Here, we report not only the first neuroimaging study, to our knowledge, to examine the responses of currently depressed subjects to food stimuli, but also the first to examine differential brain activity in those who report depression-related appetite increases versus decreases. Our findings demonstrate that food cues elicit potentiated activity in reward circuitry of individuals whose depression is associated with increased appetite. In contrast, food cues elicit attenuated activity in the interoceptive circuitry of individuals whose depression is associated with decreased appetite. These differences in brain activity to food cues may thus serve as novel phenotypic biomarkers of depression subgroups with distinct pathophysiologies and potentially illuminate the path toward new interventions targeting the development of depression-related obesity and its concomitant illnesses.