The relationship between cumulative antipsychotic exposure and mortality among patients with schizophrenia has been hotly debated during the past decade. Especially in Europe, public media have widely claimed that the shorter life expectancy of patients is attributable to long-term exposure to antipsychotics and that the use of such medications should be avoided whenever possible (
1). Thus far, only two published studies have described the relationship between risk of death and cumulative antipsychotic dose in representative patient populations (
2,
3), but those studies did not control for the effect of concomitant psychotropic medications. Another study investigated cardiac mortality and cumulative exposure to antipsychotics (
4), but it included only patients who had more than one prescription for oral thioridazine, haloperidol, risperidone, or clozapine. Since patients with minimal or no antipsychotic treatment were excluded and the control group consisted of patients with glaucoma or psoriasis, it was not possible to draw any conclusions on whether cardiac mortality was associated with antipsychotic use or lack of use. Given that a large proportion of patients with psychotic illness are also exposed to antidepressants and benzodiazepines (
5), it is somewhat surprising that the consequences of cumulative exposure to these medications have not drawn much attention. Thus far, one study has investigated mortality and current antidepressant use in schizophrenia (
6), reporting a significantly decreased risk for suicide and a nonsignificant relationship between overall mortality during current antidepressant use compared with no use. To date, the relationship between benzodiazepine use and mortality has been studied in two publications (
6,
7). In both studies, current benzodiazepine use was associated with an increase of 80%−90% in mortality. Since psychotropic medications may have fatal adverse events that manifest after long-term chronic exposure, it is also important to know the risk of death associated with cumulative use of these other drugs. To our knowledge, no such studies on quantitative exposure to antidepressants or benzodiazepines have been conducted. Therefore, we studied the relationship between cumulative antipsychotic, antidepressant, or benzodiazepine exposure and mortality in Sweden by using prospectively collected nationwide databases.
Method
We used nationwide register data to conduct a prospective population-based cohort study of patients with schizophrenia, as previously described (
2). This research project was approved by the Regional Ethics Board of Stockholm (decision 2007/762–31).
Study Population
The cohort participants were identified from two nationwide health care registers of 7,040,632 people 17–65 years of age who lived in Sweden in 2005 according to Statistics Sweden. In Sweden, all individuals have a unique personal identity number, which makes it possible to track and identify each individual in all databases. One of the two registers used was the National Patient Register (maintained by the National Board of Health and Welfare), which contains information on all inpatient care since 1988 and visits to specialized outpatient units since 2001 (only larger cities having specific units for patients with psychosis). From that register, we selected patients who had received any health care for any psychosis (ICD-10 codes F20–F29) before Jan. 1, 2006 (N=17,919) and who had received a diagnosis of schizophrenia (ICD-10 code F20) by the end of the follow-up (Dec. 31, 2010). The other database used was the MiDAS register (maintained by the Social Insurance Agency of Sweden), which contains information on people granted a disability pension. From that register, all people on a disability pension due to schizophrenia (ICD-10 code F20) before Jan. 1, 2006, were included. Altogether, 21,492 individuals were identified (corresponding to a prevalence of 0.34%). The follow-up began on Jan. 1, 2006, and ended on Dec. 31, 2010. Thus, the follow-up period was 5 years for this patient cohort.
Mortality and Covariates
Information on all prescribed and dispensed drugs from pharmacies in Sweden was obtained from the Prescribed Drug Register (maintained by the National Board of Health and Welfare). We selected all antipsychotics dispensed from 2006 through 2010 except lithium, according to the Anatomical Therapeutic Chemical (ATC, code group N05A; see the ATC/DDD Index,
http://www.whocc.no/atc_ddd_index/). Antidepressants included ATC group N06A, and benzodiazepines ATC groups N05BA, N05CD, N05CF, and N03AE01. The cumulative exposure of antipsychotics was estimated by using the defined daily dose (DDD; see the ATC/DDD Index for the definition of DDD for each medication). First, we calculated the sum of dispensed medication as DDD. Next, we divided the sum by the length of follow-up in days, of which the days spent in a hospital were subtracted, because antipsychotic medications that may be used in hospitals are not recorded in the Prescribed Drug Register. The identified schizophrenia patients were categorized into four DDD groups: 1) no antipsychotics, antidepressants, or benzodiazepines during the follow-up, 2) small doses or occasional use (0–0.5 DDD/day, noninclusive), 3) moderate doses (0.5–1.5 DDD/day, inclusive), and 4) high doses (>1.5 DDD/day), as in our previous study (
2). For example, one DDD is 5 mg for risperidone, 20 mg for fluoxetine, and 10 mg for diazepam.
Date and cause of death were obtained from the Causes of Death Register (maintained by the National Board of Health and Welfare). Death certificates are generally written by ordinary physicians, and by forensic specialists if death is sudden or suspect. The documented causes of death are considered reliable (
8). The following specific causes of death were investigated in addition to overall mortality: cardiovascular diseases (ICD codes I00–I99) and suicide (ICD codes X60–X84).
Demographic characteristics were obtained from the LISA register (maintained by Statistics Sweden). To study the effects of clinical and sociodemographic characteristics, patients were described as receiving outpatient treatment (number of visits during 2001–2005, a proxy marker for treatment adherence) or inpatient treatment for psychosis (hospital days during 1988–2005, a proxy marker for disease severity). In addition, the association of mortality with disease duration was assessed by calculating the time from the first diagnosis of psychosis in the registers until the start of follow-up. To study the putative survival bias, we also conducted a sensitivity analysis among first-episode patients. This cohort has been described in detail previously (
2).
Statistical Analysis
Cox regression analysis was used to compare overall mortality and mortality due to specific causes in different DDD groups after verifying that the proportional hazard assumption was met. Next, Cox regression analyses were used to compare the DDD groups. Initially, we checked to see if the interactions of DDD group by demographic characteristic or by clinical characteristic were statistically significant. Statistical analysis of data was performed with the R software package, version 3.11.
Results
The demographic and clinical characteristics of the cohort are summarized in
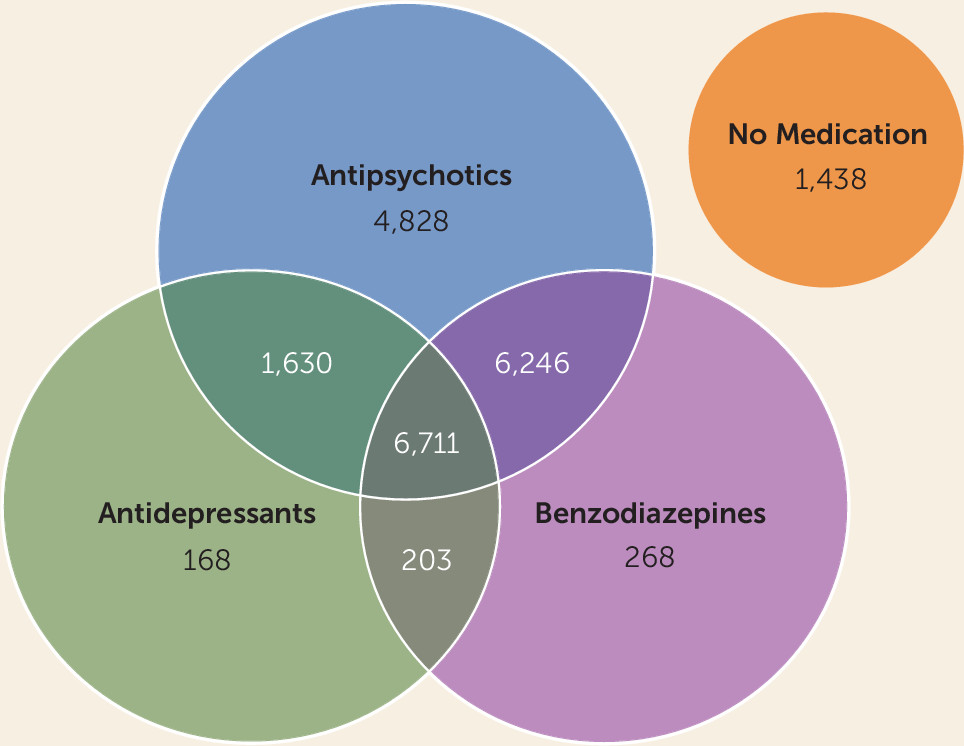
Table 1, and the proportions of those using antipsychotics, antidepressants, and benzodiazepines are listed in
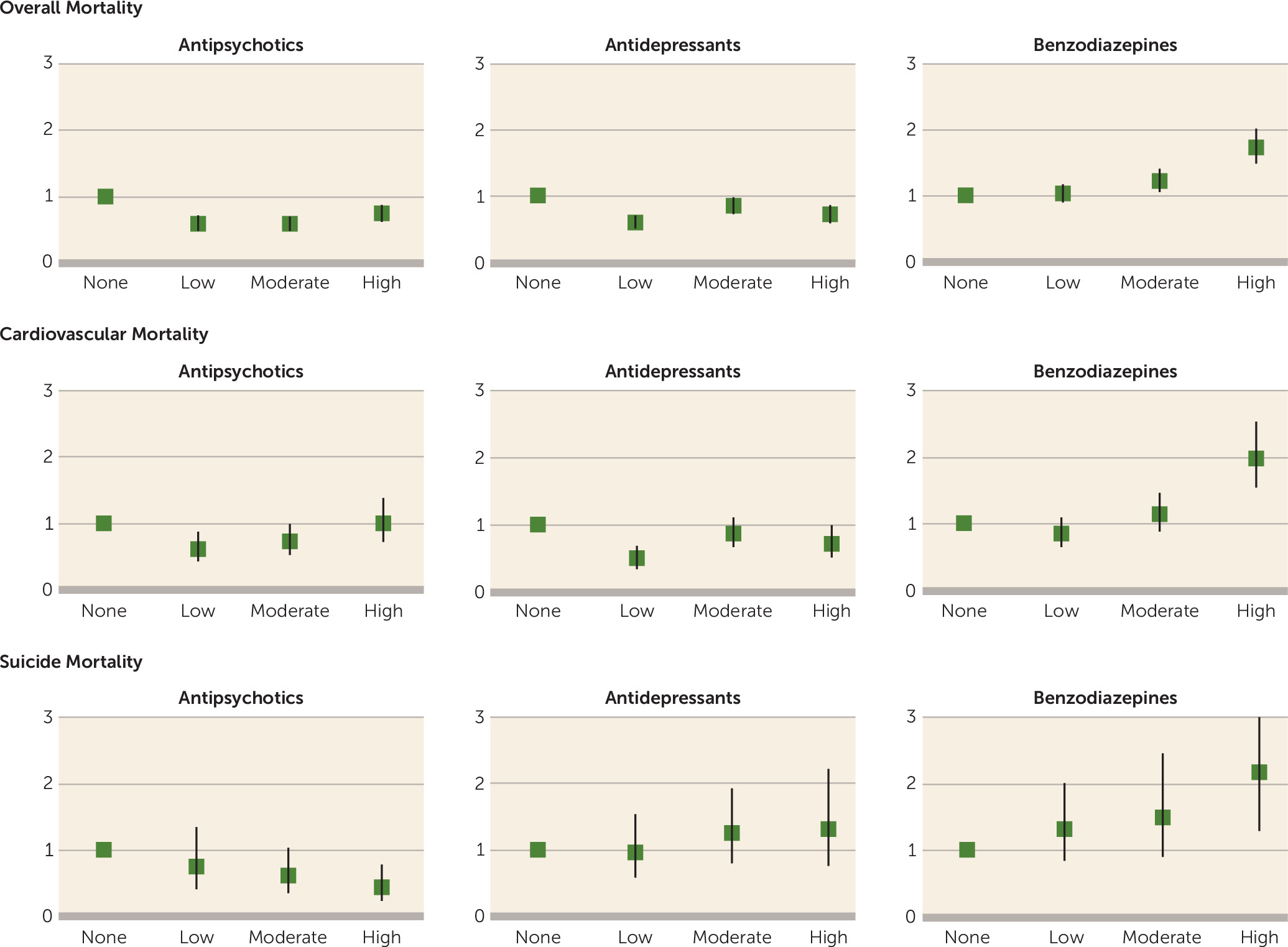
Figure 1. A total of 1,591 (7.4%) patients died during the 5-year follow-up. Compared with 214,670 age- and gender-matched persons from the general population, the mortality of the cohort was 4.8-fold higher (95% CI=4.5–5.1). The most common specific cause of death was cardiovascular disease (N=520, 32.7%), followed by neoplasms (N=262, 16.5%), respiratory diseases (N=175, 11.0%), and suicide (N=151, 9.5%). No significant interactions were observed for DDD group by demographic or clinical characteristics. The mortality rates and adjusted hazard ratios for the different antipsychotic, antidepressant, and benzodiazepine exposure groups are listed in
Table 2 and
Figure 2. Any degree of exposure to antipsychotics or antidepressants was associated with a lower overall mortality compared with no use. The opposite was observed for benzodiazepines, and high exposure was associated with a 74% higher risk of death compared with no use. In the subpopulation of patients with moderate antipsychotic use (N=8,468) (
Table 2), high exposure to antidepressants was associated with lower mortality (hazard ratio=0.73, 95% CI=0.53–1.01), and high exposure to benzodiazepines with higher mortality (hazard ratio=1.49, 95% CI=1.13–1.96), compared with no use of these medications.
When moderate exposure was used as the reference instead of no use, high-dose antipsychotic exposure was associated with 27% higher mortality, high-dose antidepressant exposure 16% lower mortality, and high-dose benzodiazepine use 41% higher mortality. The mortality rates and the number of visits to health care services among patients with different combinations of antipsychotic and benzodiazepine exposure are listed in
Table 3. Patients with high benzodiazepine exposure had the highest mortality and the most frequent visits to health care services.
The results for the most common specific cause of death, cardiovascular disease, were analogous to overall mortality, with the exception of equal mortality for high antipsychotic exposure compared with no use. Concerning suicide mortality, the only statistically significant findings were a higher risk of death with high benzodiazepine exposure and a lower risk of death with high antipsychotic exposure.
In a sensitivity analysis among first-episode patients (1,230 patients, 45 deaths), the only statistically significant findings were a decreased risk of death for low (adjusted hazard ratio=0.47, 95% CI=0.23–0.97) and moderate (adjusted hazard ratio=0.18, 95% CI=0.07–0.45) exposure to antipsychotics, and increased mortality for moderate (adjusted hazard ratio=2.72, 95% CI=1.10–6.73) and high (adjusted hazard ratio=3.86, 95% CI=1.41–10.55) exposure to benzodiazepines.
Discussion
To our knowledge, this is the first study to investigate the association between mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines. The results indicate that any amount of antipsychotic and antidepressant usage is associated with overall mortality rates 15%–40% lower compared with no use of these medications. In contrast, benzodiazepine exposure revealed a clear dose-response curve for mortality, where high exposure was associated with a 70% higher risk of death compared with no use. While it is probable that patients who need additional benzodiazepine treatment have more anxiety, insomnia, and depressive symptoms than other patients, it is also likely that high-dose chronic use of benzodiazepines, in violation of treatment guidelines, may have become an iatrogenic cause for excess mortality in this patient population. On the other hand, patients who need add-on antidepressant treatment may also suffer from anxiety and depressive symptoms, which increase cardiovascular morbidity, suicidal behavior, and mortality (
9–
13). Therefore, we may assume that the decrease in mortality associated with antidepressant use would have been even greater if we had been able to eliminate confounding by indication by adjusting for the severity of clinical symptoms, in addition to treatment history and sociodemographic factors.
Our results on cumulative use of antidepressants are in line with the results obtained from a Finnish first-episode patient cohort, which showed a trend toward lower mortality (hazard ratio=0.57, 95% CI=0.28–1.16) during current use compared with no current use of antidepressants (
6). The lower overall mortality related to antidepressant use was partly attributable to lower cardiovascular mortality. This finding may be partly explained by the anticoagulant and anti-inflammatory effects of the most widely used antidepressants (such as selective serotonin reuptake inhibitors), thus leading to lower risk of cardiac infarctions (
14). We did not observe any decrease in suicide mortality as a function of cumulative antidepressant exposure, as we did in the comparison of current use and no current use in our previous study (
6). This may be explained by the time of death, since those patients with high or moderate exposures may have committed suicide when they had discontinued their medication. Unfortunately, it was not possible to conduct additional current-use analysis in this database.
Our finding on mortality and cumulative exposure to benzodiazepines is similar to results from two previous studies on current versus no current benzodiazepine use (
6,
7). The associations between excess overall mortality, suicide mortality, and cardiovascular mortality were all alike concerning high exposure to benzodiazepines. Treatment guidelines recommend that benzodiazapines not be used for longer than 1 month to avoid tolerance, dependence, and dose escalation. Therefore, it is alarming that one-third of the population of patients with psychosis in Sweden had used on average more than 0.5 DDD/day of benzodiazepines, which is equal to more than 5 mg of diazepam or 25 mg of oxazepam every day during the 5-year follow-up. As common as long-term benzodiazepine use was in our study population, the literature indicates that it is probably more common among patients with schizophrenia in other developed countries, such as the United States (
15,
16).
The association between benzodiazepine exposure and mortality may be explained by several mechanisms. Although long-term benzodiazepine use may be a marker for more severe illness and coexisting substance abuse, it is also plausible to assume that prescribing of high doses for long periods may lead to tolerance and dose escalation. This could result in fatal interactions with concurrent use of alcohol and illicit drugs, and worsening of polysubstance dependence may result in a less healthy lifestyle in general. In addition, high-dose benzodiazepine use may also contribute to daytime sedation and proneness to accidents.
The results on cumulative exposure to antipsychotics resembled those of Cullen et al. (
3), as well as those obtained in our preliminary study (
2), but the effect sizes changed slightly when we also adjusted for the effect of cumulative exposure to antidepressants and benzodiazepines. In particular, the mortality related to high-dose antipsychotic use decreased when we adjusted for the effect of concomitant high-dose benzodiazepine use. These results imply that both excess overall mortality and cardiovascular mortality in schizophrenia are attributable to factors other than antipsychotic treatment when used in adequate doses. This finding is in line with results obtained from Finnish (
17–
19), Swedish (
2,
20), and Danish (
7) nationwide cohorts, as well as with those from a U.S. Medicaid cohort (
3), all of which showed lower mortality during current antipsychotic use compared with no use. To our knowledge, these cohort studies are the only sufficiently large studies comparing mortality during antipsychotic use and no use among patients with schizophrenia, and they all show similar results. The results from the first-episode patients resembled those from the main cohort, but because of the lower number of subjects and a shorter follow-up time, the only statistically significant findings were a decreased risk of death for low and moderate exposure to antipsychotics and increased mortality for moderate and high exposure to benzodiazepines.
It is possible that patients who have not used antipsychotics, antidepressants, or benzodiazepines are so mildly ill that they do not need any medication, and that those patients with high-dose use are the most severely ill. Another possibility is that those patients not using any medication are those with the poorest insight, declining all treatment and avoiding contact with health care services. Although we adjusted for the effect of frequency of voluntary outpatient visits to health care services (a proxy marker for treatment adherence), there may still be residual confounding, which has to be taken into account when considering causality. When moderate exposure was used as the reference instead of no use, high-dose antipsychotic use was associated with a 27% higher overall mortality, and high-dose benzodiazepine use, 41%, whereas high-dose antidepressant use was associated with a 16% lower mortality. Even this kind of comparison indicates that the high-risk patients are especially those using long-term high-dose benzodiazepine treatment. Our results revealed that patients with high-dose benzodiazepine exposure had the most frequent treatment contacts with the health care system, which implies that the putatively higher degree of physical illness in this patient group cannot be explained by fewer contacts or less access to health care services.
Currently, and with scant scientific data, adverse events of antipsychotics are considered the main issue in excess mortality, but it appears that the lack of antipsychotic usage is associated with the highest rates of overall and cardiovascular mortality. It is important to realize that although monitoring of patients with moderate or high-dose antipsychotic treatment is relevant, it is essential to focus the preventive interventions on those patients who have an even higher risk of death, that is, patients not using antipsychotics and patients using high doses of benzodiazepines. The highest risk was observed for first-episode patients with high-dose benzodiazepine use, with an almost fourfold higher mortality compared with the majority of patients with no benzodiazepine use. The results of this study suggest that these characteristics of patients are useful markers for high risk of death. Irrespective of the causal mechanisms, these patient groups should receive close monitoring and active treatment of their physical and mental health conditions, suggesting a radical paradigm shift in the treatment of schizophrenia.