Depressive and anxiety disorders are associated with shorter leukocyte telomere length (LTL), an indicator of cellular aging (
1). This might help explain why depressed or anxious persons have increased onset risks of several aging-related somatic conditions, such as cardiovascular disease, diabetes type II, and obesity (
2,
3). One of the proposed pathways is that various biological abnormalities in depressive and anxiety disorders, such as a dysregulated immune system or increased oxidative stress (
4,
5), may lead to cellular damage, including shortened telomere length. Another less-explored explanation is that short telomere length might be a risk factor for persons to develop a depressive or anxiety disorder. Alternatively, an underlying third factor, such as genotype or lifestyle, may make persons vulnerable for both short telomeres and depressive or anxiety disorders (
6). As human life expectancy increases, research into aforementioned pathways becomes increasingly important. A better understanding of biological mechanisms may yield novel interventions that prevent aging to be inevitably accompanied by aging-related somatic diseases and disability.
LTL has emerged as an indicator of biological or cellular, rather than chronological, age, since 1) it decreases progressively with every cell division (unless counteracted by sufficient activity of the ribonucleo-protein enzyme, telomerase) and thus with age (
7); 2) it reflects damage accumulated over the years by lifestyle, psychological stress, and cytotoxic environments (
8); and 3) it correlates and predicts the incidence of numerous serious medical illnesses (
9). Several cross-sectional studies, with a few exceptions (
10–
12), found shorter LTL in depressed patients compared with control subjects (
13–
16), including investigations in the present sample (
17). This was further confirmed by a recent meta-analysis that found significant effect sizes for the association between depression and shorter LTL (
1). Also, although less often studied, shorter LTL was found in anxiety disorder patients compared with control subjects (
18,
19). A major shortcoming of most studies published until now is their cross-sectional nature, limiting the ability to draw any inferences regarding cause and effect. Only two studies employed longitudinal designs: Hoen et al. (
13) found that depression status in 608 heart disease patients did not predict 5-year change in LTL, while Shalev et al. (
20) found that 193 men with a depression or anxiety disorder between the ages of 26 and 38 showed greater LTL decrease in that time frame compared with 226 healthy men, but this association was not present in women. In summary, it remains unclear to what extent possible telomere shortening is reversible when persons recover, whether chronicity of a disorder leads to accelerated shortening of LTL over time, whether there are common (genetic) risk factors for both, or, alternatively, whether short LTL is a vulnerability factor that may antecede the development of depressive and anxiety disorders. Longitudinal data are needed to explore these questions.
The present study examines the 6-year longitudinal relationship between LTL and depressive and anxiety disorders using a large sample (N=2,936). Objectives were to examine whether presence of diagnoses and symptom severity were consistently associated with LTL over time and whether changes in depressive and anxiety disorder characteristics (course, duration, and severity) corresponded with changes in LTL.
Discussion
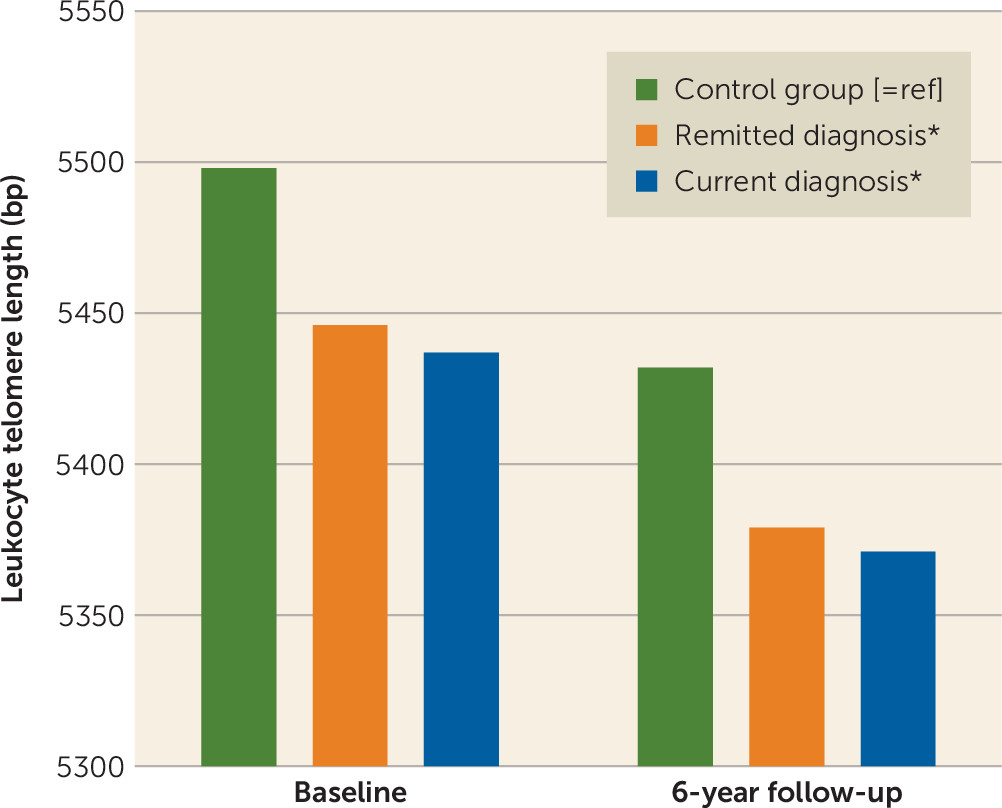
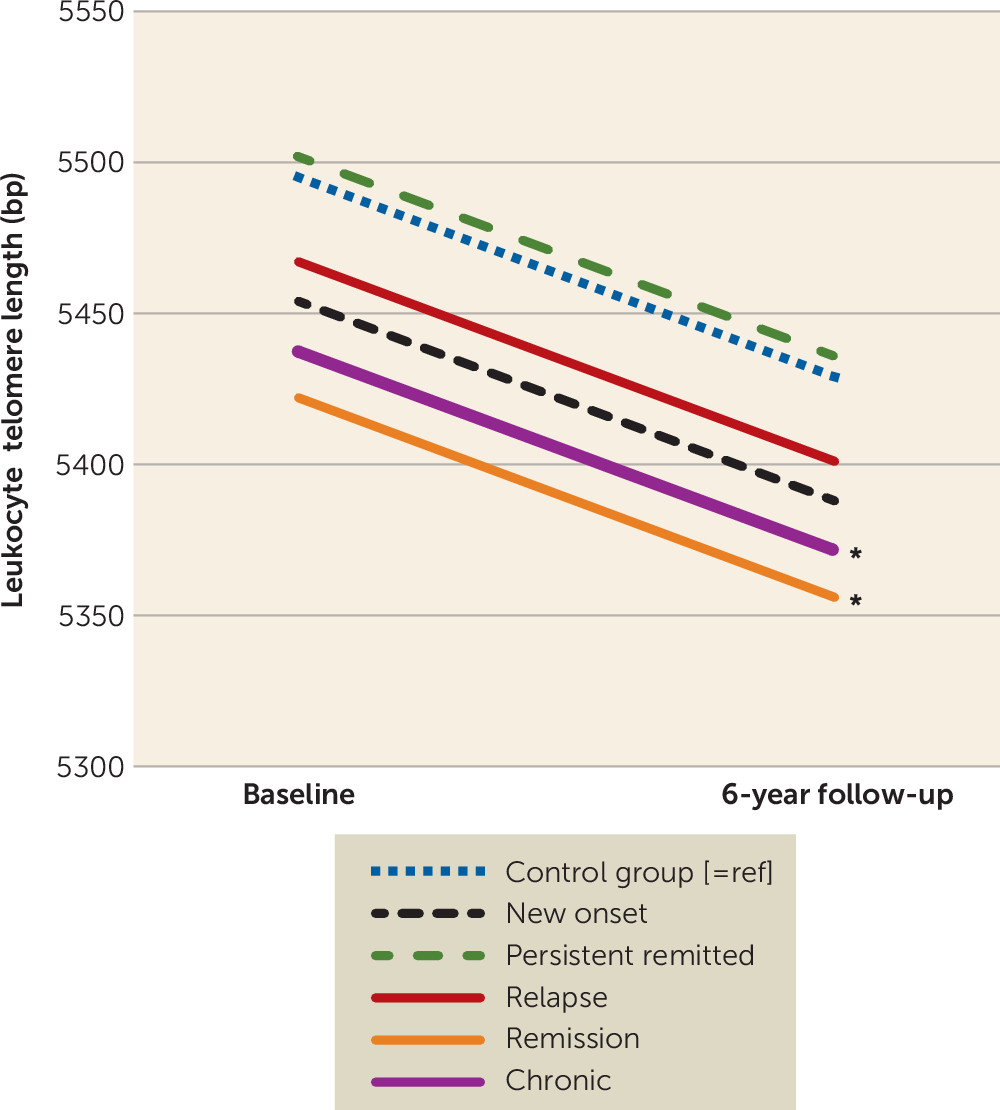
This large study with 6-year longitudinal data demonstrated that persons with a lifetime depressive or anxiety disorder had consistently shorter LTL than nonpsychiatric control subjects at two time points, irrespective of the current diagnosis status. In line with a dose-response association, we found negative associations between LTL and the severity of depressive and anxiety symptoms, indicating that the most severe patients had the shortest telomeres. In different analytical approaches, diagnosis status or changes in diagnosis status did not correspond with changes in LTL. First, persons with depression or anxiety disorder at baseline did not show accelerated 6-year LTL attrition compared with control subjects. Second, six groups based on the course of depressive and anxiety disorders over four assessments (i.e., control subjects, persistently remitted persons, persons with new onset, persons with relapse/remission, chronic persons) showed no difference in telomere attrition rate. Third, changes in symptom severity, duration of symptoms, and number of study waves with a diagnosis did not correlate with change in LTL. Overall, these findings do not suggest a dynamic relationship: existing differences in LTL did not become larger or reduced over time depending on, for example, whether a person developed more severe, chronic symptoms or recovered.
Our results point toward a between-person rather than a within-person relation of depressive and anxiety disorders and LTL, since a person’s LTL did not change along with changes in that person’s psychopathology. In other words, persons with a lifetime diagnosis were found to have shorter LTL, which represented a difference in “cellular age” of 4 years (remitted diagnosis) and 4.5 years (current diagnosis), and this was irrespective of whether they relapsed or recovered from a disorder. This suggests that either 1) shorter LTL is indeed a consequence of depressive and anxiety disorder-related physiological disturbances, leaving a long-term (cellular) scar, or 2) persons with short LTL, possibly due to genetic heritability (
6,
32), are more prone to developing a depressive or anxiety disorder, and LTL attrition rate is not necessarily accelerated when those persons meet a disorder diagnosis. Our results resemble those of Hoen et al. (
13) in 206 heart disease patients with depression. Shalev et al. (
20) did find greater LTL decrease in 193 men between the ages of 26 and 38 with one or more depressive or anxiety disorders (depression and generalized anxiety disorder) in a 12-year time frame compared with 226 men without a diagnosis, but this association was not reported for women. Substantial study differences might account for the conflicting outcomes regarding LTL change: Shalev et al. had a younger (mean age: 26 years compared with 42 years at baseline) and smaller (N=758 compared with N=1,860) study sample and a longer follow-up period (e.g., 12 years compared with 6 years). Moreover, unlike Shalev et al., our study assessed LTL at baseline and the 6-year follow-up in different batches, which might have caused noise between the two time points. Furthermore, Shalev et al. did not report whether the number of phases with an internalizing disorder increasingly affected LTL change and whether the effect was still significant after adjustment for lifestyle variables.
It is important to note that we found that LTL at baseline was negatively associated with LTL change, indicating that those with short LTL at baseline had a higher chance of lengthening, and vice versa (
29). However, despite this compensatory effect that is possibly due to an internal telomere homeostasis system (
33), we still found that persons with a current and remitted depressive or anxiety disorder had consistently shorter LTL over the two time points. Something else to consider is that LTL shortening might not be limited to diagnostic categories, and it may be possible that LTL reflects underlying pathophysiological processes that surpass traditional diagnoses (
8). Evidence for this comes from studies that confirm shorter LTL among other psychiatric cases, such as those with schizophrenia (
34) or PTSD (
35).
The major strengths of this study are its large sample size, including well-characterized patients with current and remitted depressive and anxiety disorders, as well as healthy control subjects. Moreover, the sample had a wide age range, and important covariates such as health and lifestyle variables were assessed at multiple time points. Also, LTL was measured reliably with qPCR, and interassay coefficients of variation were sufficiently low. These strengths allowed us to thoroughly examine the longitudinal relation between LTL and depressive and anxiety disorders. However, some limitations of this study should also be noted. First, it should be noted that results of this observational study did not take into account whether persons recovered spontaneously or as a result of psychological or pharmacological treatment, whereas this may actually have an impact on LTL or its regulatory mechanisms (
30); possible short-term changes due to treatment may thus not have been captured. Hence, although results of this study are not suggestive of reversibility of LTL shortening after recovery from a depressive or anxiety disorder, multiple in vitro (
36,
37) and in vivo (
38) studies have systematically shown that such reversibility is indeed possible, possibly even as a result of psychotropic treatment (
37,
39,
40). Furthermore, LTL from baseline and the 6-year follow-up was measured 2 years apart, which could have caused noise between the time points. To adjust for possible systematic differences, samples from both time points were rerun together and LTL at follow-up was converted accordingly. Next, as in most studies, we used leukocytes for TL measurement, which is a validated and often-used indicator for cellular aging. A limitation of using average leukocyte TL is that it consists of different cell types. A recent study found different TL change rates for T-cells, B-cells, and monocytes, which makes it difficult to distinguish whether LTL differences are due to actual shortening/lengthening or rather to a redistribution of cell types (
41). However, LTL has been found to have a rather high consistency and rather similar shortening rates across various tissues (i.e., buccal cells, skeletal muscle, skin, and subcutaneous fat) (
42), but not necessarily all tissues. This suggests that the results of studies conducted in leukocytes are to some extent generalizable to other cell types. Another limitation is that telomerase activity has not been measured, which would be interesting in future research, since telomerase activity may play an important role in the maintenance of LTL, as suggested by several intervention studies (
30), and may even mediate beneficial effects of psychotropic medication (
43).
In conclusion, this 6-year longitudinal study showed that LTL was consistently shorter for those with a lifetime depressive or anxiety disorder diagnosis, especially among those with the most severe symptoms (dose-response). Importantly, we found that LTL differences over 6 years did not become more pronounced or diminished as a consequence of incidence, remission, or chronicity of depressive or anxiety disorders. While it should be noted that possible short-term treatment effects might not have been captured in this observational study, absolute LTL changes were not related to changes in diagnosis status, symptom severity, or duration. This indicates a static, nondynamic relationship between depressive or anxiety disorders and short LTL and suggests the absence of a direct within-person relationship, since changes in psychiatric characteristics did not correspond with changes in LTL. Future research should elucidate whether short leukocyte telomere length is a long-term consequence or a pre-existing risk factor for the development of depressive or anxiety disorders.