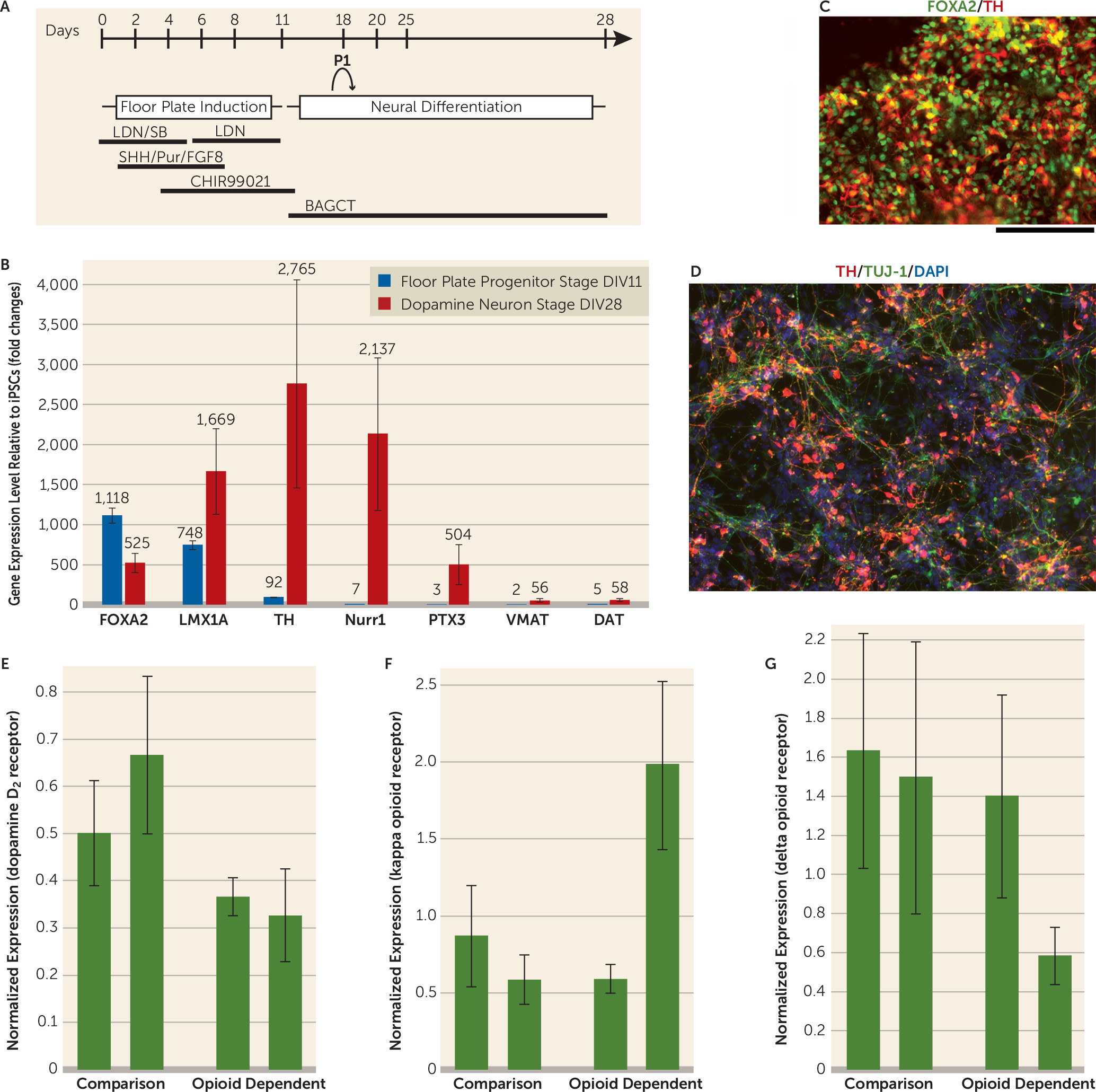
The dopaminergic system has been implicated to have an important role in addiction. However, human dopaminergic neurons from drug-dependent subjects were not available for study until recent developments in iPSC technology. Our objective was to study human dopaminergic neurons derived from drug-dependent individuals. The iPSCs were derived from four subjects (two opioid-dependent subjects and two comparison subjects) as shown in
Figure 1. We successfully generated midbrain dopamine neurons that expressed forkhead box protein A2,
LIM homeobox transcription factor 1 alpha, tyrosine hydroxylase, the dopamine D
2 receptor, the Nuclear receptor related 1 protein, the vesicular monoamine transporter 2, and the dopamine transporter (
Figure 1). All iPSC lines generated dopamine neurons that released dopamine detectable by high-performance liquid chromatography (ranging from 10.83 nm [SD=1.89] to 60.52 nm [SD=4.83]). There was no apparent difference in dopamine differentiation efficiency between cell lines derived from the comparison subjects and the opioid-dependent subjects with regard to the percentage of cells positive for tyrosine hydroxylase. Importantly, there were different expression levels of D
2 receptors in the comparison group’s iPSC-derived dopamine neurons compared with the opioid-dependent group’s iPSC-derived dopamine neurons. Specifically, our results showed lower expression levels of D
2 receptors in both of the opioid-dependent iPSC lines compared with the dopamine neurons derived from the comparison group’s iPSC lines (relative dopamine D
2 mRNA levels were equal to 0.37 [SD=0.04] and to 0.33 [SD=0.09] for the opioid-dependent dopaminergic neurons, and levels were equal to 0.50 [SD=0.11] and to 0.67 [SD=0.17] for the comparison dopaminergic neurons). These results are consistent with previous neuroimaging findings showing that D
2 receptor levels are lower in opioid-dependent subjects and in a variety of other addictions (
1,
2).
We also examined the expression of different classes of opioid receptors. Previous reports in animal studies suggest that mu-receptor-specific opioid agonists might excite dopamine neurons only through indirect mechanisms (
3). Our data showed that mu opioid receptors were not detected in our differentiated human dopamine neurons derived from iPS cells, supporting this hypothesis in human dopamine neurons. In contrast, kappa and delta opioid receptors were detected in our human dopamine neuronal cultures from all four iPSC lines. This is consistent with reports that kappa opioid agonists can directly inhibit midbrain dopamine neurons and that delta receptor activation in the substantia nigra or ventral tegmental area results in protection in Parkinson’s disease models and protection against alcohol addiction (
4,
5). Although iPSCs have been used in multiple disease models, including amyotrophic lateral sclerosis, multiple sclerosis, and Parkinson’s disease (
6), the use of iPS cell technology for drug abuse has been very limited. Our data suggest that human iPSC-derived dopamine neurons may serve as a useful model to study human dopamine neurons in vitro and to compare differences between drug-dependent and comparison subjects. In the future, it might also be useful to test toxicity and pharmacological responses to potential treatments on an individual basis.