Current treatment algorithms for the management of psychosis do not take into account the substantial interindividual variability in response to antipsychotic drugs. Clinicians have no alternative but to choose antipsychotics based on trial and error, often resulting in prolonging patients’ suffering. What if a brief brain scan or a blood test prior to starting an antipsychotic were sufficient to provide an accurate measure of how likely an individual was to respond to a specific medication and could thus guide treatment decisions? Two articles in this issue report on advances toward individualized medicine in the treatment of psychosis.
In order to develop a biomarker of treatment response with clinical utility, researchers must first demonstrate a robust association between the biomarker and clinical outcome, and then must replicate these findings in independent cohorts. Based on converging evidence implicating the striatum as critical to antipsychotic response, Sarpal et al. (
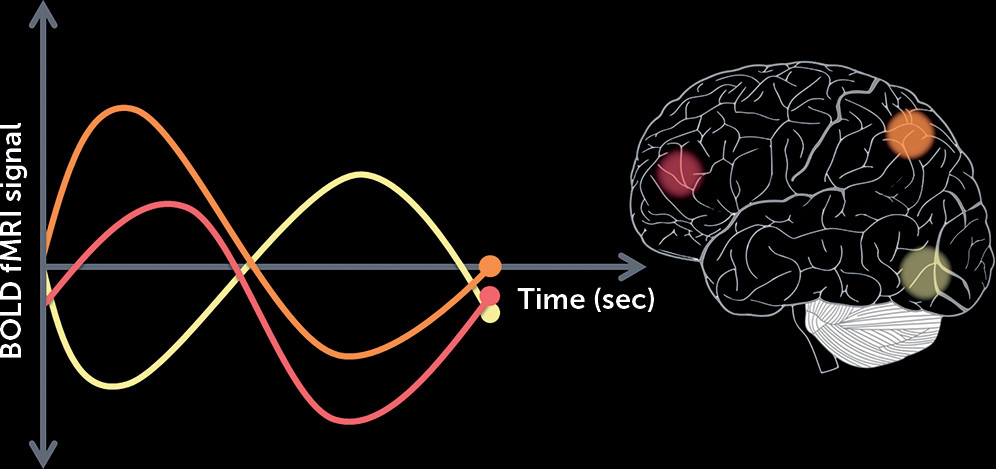
1) hypothesized that functional connectivity between the striatum and other brain regions could be established as a biomarker indexing the likelihood that an individual will respond to treatment. Functional connectivity leverages the temporal coherence of low-frequency fluctuations in the blood-oxygen-level-dependent signal between remote brain regions obtained during resting-state functional MRI to allow inferences on coordinated brain activity (
Figure 1). The authors obtained a 5-minute resting-state scan in a group of first-episode psychosis patients (the “discovery cohort”) at the initiation of a 12-week randomized trial with either risperidone or aripiprazole and determined the response to treatment at the end of the trial. By regressing treatment response values with baseline functional connectivity, striatal connectivity to 91 brain regions was identified to be predictive of subsequent response to treatment. For each participant, a striatal connectivity index was calculated by summing the strength of the functional connectivity between the striatum and each of these regions. This index was found to be significantly different between treatment responders and nonresponders. To validate this putative biomarker, similar resting-state scans were then obtained in a diagnostically heterogeneous group of patients who were hospitalized for an acute exacerbation of psychosis (“the generalization cohort”). The same striatal connectivity indices identified in the discovery cohort predicted treatment response in the generalization cohort, with 80% sensitivity and 75% specificity. Replicating this finding in such a diverse group speaks loudly about the potential for future clinical applications.
Importantly, since schizophrenia is now conceptualized as a brain dysconnectivity syndrome (
2), use of methods that included searching the whole brain for striatal functional connectivity predictive of treatment response maximized the amount of treatment-related variance captured. Interestingly, these biomarkers were able to distinguish responders from nonresponders irrespective of clinical characteristics such as illness duration, exposure to medication prior to scanning, and severity of psychosis, all features that potentially have an impact on striatal functional connectivity. Likewise, our group has reported (
3) that the resting-state functional connectivity of the ventral tegmental area/midbrain (the source of dopaminergic projections thought to be critical for antipsychotic action) prior to treatment, when patients were unmedicated, was also predictive of subsequent treatment response. Taken together, the functional organization of the brain before treatment appears to be set in a way that does or does not favor treatment response. But how close are we to being able to use these biomarkers in the clinical setting? Further characterization of these biomarkers in larger and well-characterized cohorts will be necessary before they can be implemented in routine practice.
Heide et al. (
4) report in this issue on another promising avenue toward the use of biomarkers to predict treatment response, based on the work of several antecedent studies. The propensity of antipsychotic medications to induce cardiac QT interval prolongation results from their blockade of Kv11.1 voltage-gated potassium (K
+) channels that are encoded by the gene KCNH2, which regulates heart muscle excitability and repolarization. A recently identified variant of KCNH2 (KCNH2 3.1) that increases expression of an alternatively spliced isoform Kv11.1-3.1 in the brain and modulates neuronal firing has been found to be overexpressed in postmortem schizophrenia brains (
5). This led to the hypothesis that antipsychotic action may be achieved through blockade of this brain-selective K
+ channel and that genotypes associated with increased Kv11.1-3.1 expression could be associated with better treatment response. Importantly, a pharmacogenetic study of treatment response in two independent cohorts of patients with schizophrenia revealed an association between variants in KCNH2 and treatment response (
6). In addition, a recent study reported that patients who are slow drug metabolizers, as determined by genetic variants in cytochrome P450, have a better response to treatment with risperidone than with other antipsychotic drugs (
7).
In the study reported here, Heide et al. combined pharmacogenetic and drug metabolism data to evaluate their effects on treatment response to antipsychotic. First, they report that while paliperidone and other atypical antipsychotics had similar potencies for in vitro blockade of two isoforms of the K+ channel, the cardiac-selective Kv11.1-1A and the brain-selective Kv11.1-3.1, risperidone caused greater blockade of the latter, suggesting that this might be advantageous in terms of treatment response. Using the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) database to identify subjects of European ancestry for whom drug clearance data (estimated from measures of drug concentrations) and genotype data were available (N=362), the authors evaluated the effects of KCNH2 genotypes and drug metabolism on response to risperidone compared with other atypical antipsychotics. Three KCNH2 single-nucleotide polymorphisms (SNPs) associated with increased expression of Kv11.1-3.1 in postmortem brain were selected and three SNP diplotypes were constructed (containing either 0, 1–2, or 3–4 minor alleles) to test diplotype-by-risperidone interaction on treatment response. Drug metabolism was classified as fast, intermediate, or slow. When all patients were considered together, irrespective of KCNH2 genotype, there was no difference in treatment response between patients treated with risperidone and those treated with the other drugs. However, when they were classified according to KCNH2 genotype, patients treated with risperidone who carried the greater number of minor alleles showed significant improvement in positive symptoms compared with other drugs. Furthermore, a three-way interaction revealed that those with a greater number of minor alleles who had slow risperidone metabolism showed significant improvement in positive symptoms compared with the intermediate and fast metabolism groups. Intriguingly, the Sarpal et al. and Heide et al. studies may not be as different as they might at first appear: If increased expression of the alternatively spliced isoform Kv11.1-3.1 in the brain modulates neuronal firing, it may cause alteration in resting-state functional connectivity.
Which technique will be better at predicting treatment response in the clinical setting—a brain scan or a blood test? As antipsychotic response is likely influenced by a number of variables that may (or may not) be independent of each other, a combination of imaging, genetic, and clinical biomarkers is bound to improve treatment prediction. There are a number of statistical techniques, such as support vector machine (
8) and classification trees (
9), that allow combining multimodal data to detect which combination of modalities provides the best accuracy, in this case in terms of treatment response.
The studies published in this issue offer hope that personalized medicine is moving within reach. However, much work remains to be done to identify which combination of modalities will provide the best tools for accurate decision making in the clinical setting.
Acknowledgments
The author thanks Nina Kraguljac, M.D., for suggested edits and Jennifer Hadley, M.D., Ph.D., for the suggested graphic.