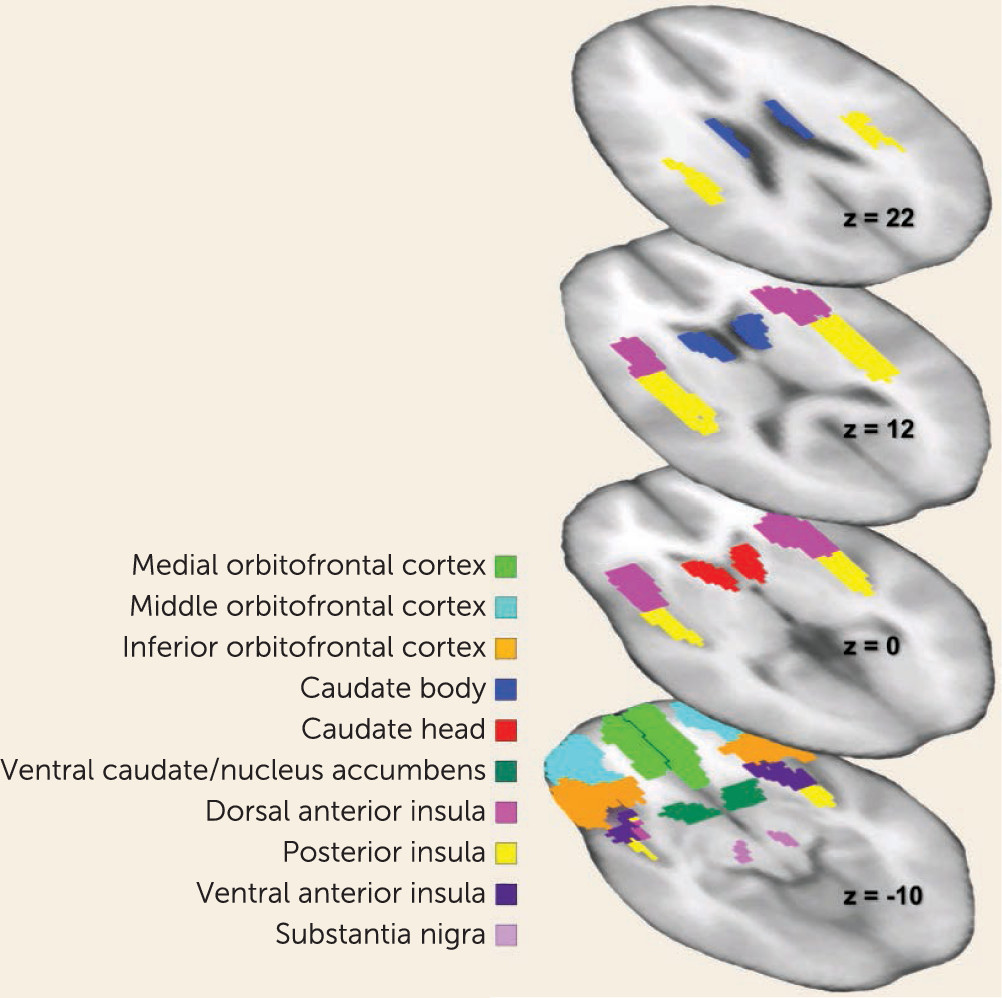
Brain imaging studies in anorexia nervosa have implicated central reward circuits that take part in the control of food intake (
3–
5). For instance, structural and functional differences in the insula have been found between patients with anorexia nervosa and healthy subjects (
6,
7). Prefrontal and striatal responses to monetary reward are also altered in adults ill with or recovered from anorexia nervosa (
8,
9). In adolescent anorexia nervosa, heightened posterior caudate response to monetary losses was associated with altered reward learning (
10). These studies provide evidence for altered reward system function in anorexia nervosa. However, neurotransmitter-based hypotheses, which are key to developing pharmacological interventions, are largely lacking.
Dopamine mediates reward learning (
11) and has been implicated in the pathophysiology of anorexia nervosa (
3,
12,
13). Midbrain dopaminergic neurons exhibit a phasic burst when an unexpected reward is received (positive prediction error). They will then shift the signal to the onset of a conditioned stimulus that they have learned predicts reward receipt (
11). A negative prediction error (a dip in dopamine neuron activity) is evoked when the predicted stimulus association is violated (unexpected reward omission, negative prediction error). The prediction error can be modeled in a temporal difference, or reinforcement learning, algorithm. In previous studies, we found increased ventral striatum, insula, and prefrontal cortex response in adult anorexia nervosa (
11,
14,
15).
We applied this model in a novel monetary reward paradigm, modeled after the taste paradigm (
14). Specifically, we aimed to study dopamine-related brain responses independent from food stimuli in adolescent anorexia nervosa. Animal studies show that food restriction or weight loss enhances dopamine response to rewards (
16), and we expected heightened brain activity in underweight adolescent anorexia nervosa. Using a longitudinal design, we tested whether group differences would normalize with weight restoration. We expected that dopamine-model-related activation would show incomplete normalization with weight restoration. We further expected that greater brain response using the prediction error model would predict poor recovery, indicating a more severe illness and suggesting that dopamine function could be a treatment target in adolescent anorexia nervosa.
Discussion
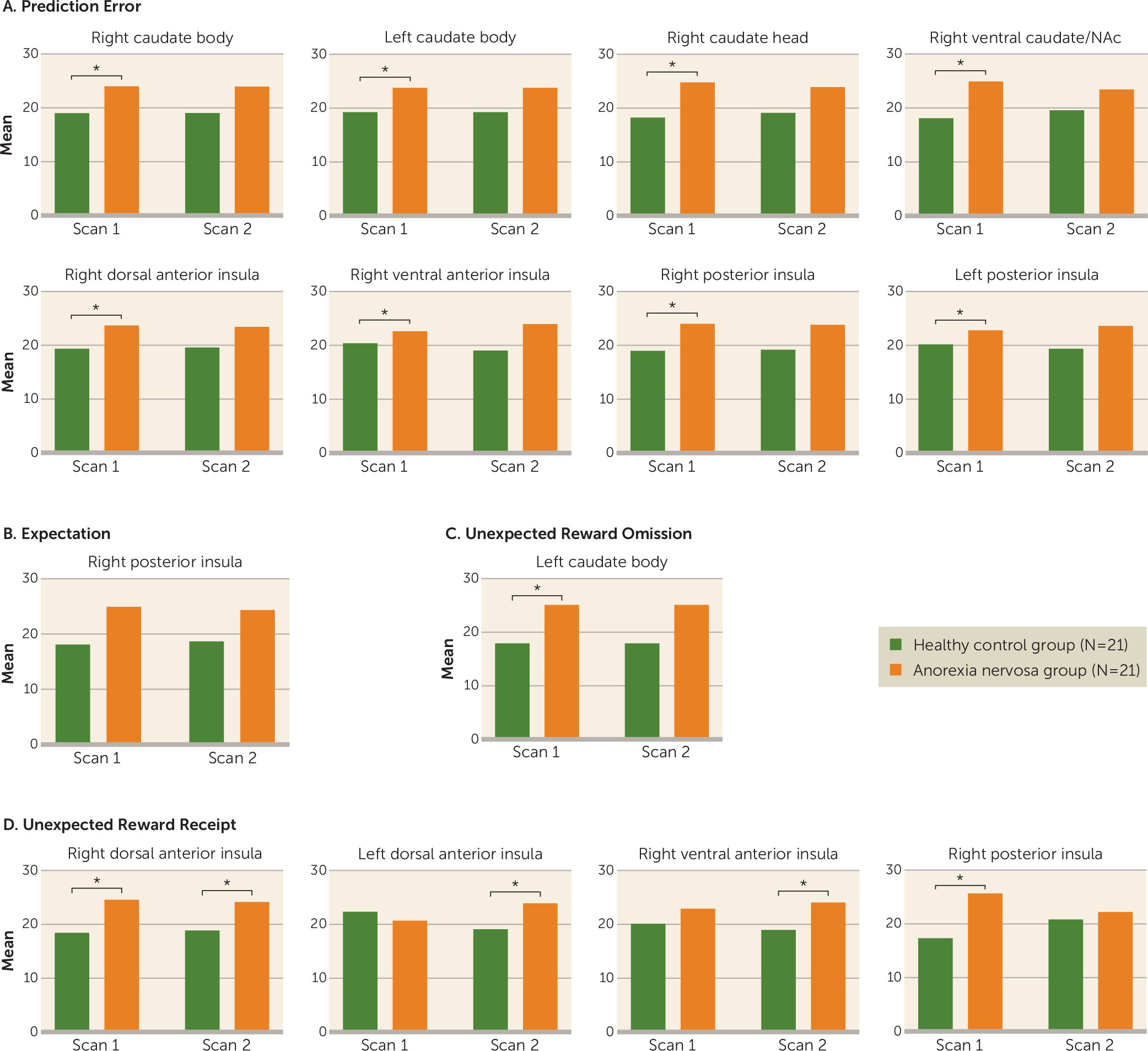
This study yielded three main findings. First, brain reward circuit responses were elevated in adolescents with anorexia nervosa in the caudate and insula during a reward learning paradigm that used a computational model for dopamine-related prediction error response. Caudate prediction error response was related to rate of weight gain during treatment and could be a neurobiological marker of illness severity to predict individual treatment needs. Second, after weight restoration, elevated prediction error responses in the striatum and insula tended to normalize but were still elevated. This result aligns with animal models showing that underweight is associated with increased dopamine-related reward system responsiveness that only partially recovers with weight restoration (
25). Third, dorsal and ventral anterior insula activations to unexpected monetary receipt were also greater at discharge in the anorexia nervosa group. Those additional results support the insula’s involvement in adolescent anorexia nervosa psychopathology even after weight restoration (
26). It remains to be seen whether these patients process positive salient stimuli differently after weeks of treatment, or whether long-term effects of low body weight on insula function during unexpected receipt of salient stimuli becomes exaggerated with weight restoration.
This monetary reinforcement learning model revealed, in adolescent anorexia nervosa, elevated striatal and insular activity (
27) comparable to prediction error taste reward results in adults (
14). The caudate and nucleus accumbens are known to respond to salient stimuli (
28) and encode prediction error signals during stimulus reward learning, which may suggest altered dopamine functioning in anorexia nervosa (
29). The insula contains the primary taste cortex and integrates body perception signals, but it also contributes to cognitive control and tracks error (
30–
32). Insula prediction error signaling has been associated with flexible behavior control, and heightened response could alter reversal learning in adolescent anorexia nervosa. This may be especially relevant when an individual must reverse maintained behavior response to anxiety-provoking food cues. Whether dopaminergic neurons contributed to greater insula signaling in anorexia nervosa is unclear, but it is likely that other neurotransmitter signaling was also involved (
32,
33).
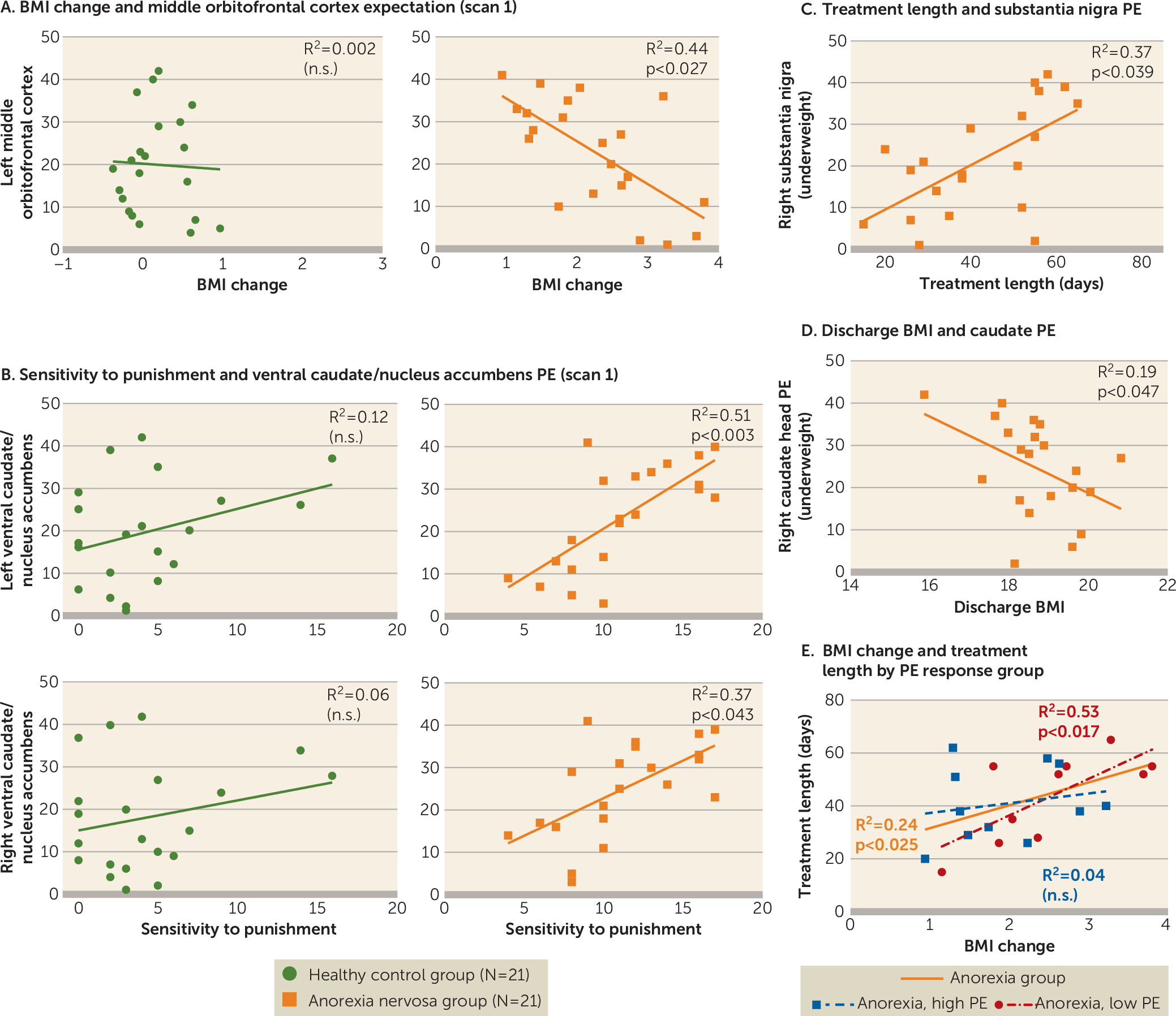
Brain response in the middle orbitofrontal cortex during reward expectation before treatment was related to weight change during anorexia nervosa treatment. Higher activation was associated with lower BMI increase, although the monetary task did not reveal group differences in orbitofrontal cortex activation. Activation in this brain region during a food cue task has been associated with a personal sense of lack of control and thoughts of guilt, as well as control over salient stimuli (
34,
35). It is possible that high response during reward stimulus expectation triggers thoughts of guilt and strengthens control over or resistance to approach of salient stimuli. Furthermore, in the anorexia nervosa group, the higher the caudate prediction error values were at the beginning of treatment, the lower the BMI was at discharge. This suggests that more severely altered brain function is reflective of more severe illness and worse outcome. A further examination of this relationship indicated that number of days in treatment predicted BMI change only in individuals in the anorexia nervosa group with relatively low prediction error signaling. In other words, individuals in the anorexia nervosa group with prediction error activity closer to that of healthy controls predictably gained weight, about 1 BMI point every 20 days; however, this did not apply to individuals with initially high prediction error brain activation. This suggests that individuals with more severely dysfunctional reward systems do not respond in the same way to the treatment regimen with therapist and family-based meal support. This finding has important implications for developing neurobiological markers of illness severity that can predict treatment needs on an individual basis. It may indicate a need to develop alternative or additional approaches for the high brain response group.
Sensitivity to punishment was elevated in adolescent anorexia nervosa, and it was positively correlated with ventral caudate/nucleus accumbens prediction error signaling when underweight, but not at discharge. Bischoff-Grethe et al. (
10) found that the posterior striatum was more sensitive to loss during a guessing game in adolescents with anorexia nervosa compared with healthy subjects, which may be in support of our finding. It should be noted, however, that our novel monetary task required participants to learn associations to predict outcomes, rather than make guesses. Elevated prediction error response could reflect high dopamine-neuronal activation in anorexia nervosa, which may drive high punishment sensitivity, especially when underweight. Harm avoidance also is typically elevated in anorexia nervosa. We further hypothesize that harm avoidance could be a functional response to excessively high sensitivity to negative salient stimuli (punishment), and thus reflects an attempt to avoid such negative experiences. In time, harm avoidance may become a learned and self-reinforcing behavior that becomes independent from weight status and high punishment sensitivity.
Interestingly, reward sensitivity scores were higher in the anorexia nervosa group but not significantly different between groups, which is in contrast to some studies but in accord with others (
36). This finding might suggest a more flexible sensitivity to reward in adolescent compared with adult anorexia nervosa, but adolescents with anorexia nervosa may nevertheless find negative salient stimuli difficult to tolerate as they navigate recovery. On the other hand, mean values for reward sensitivity in the anorexia nervosa group were comparable to those in our previous study, and the lack of significant group differences could be an effect of sample size (
37). We also found a pattern of reward expectation responses in the insula, striatum, and orbitofrontal cortex positively correlating with punishment and reward sensitivity in anorexia nervosa, although it was not significant after multiple comparison corrections. This was primarily the case for punishment sensitivity at scan 1, but with reward sensitivity at scan 2 (see Table S4 in the
data supplement). Whether there is a balance between brain reward response and sensitivity to salient stimuli that shifts from punishment to reward during weight restoration is a direction of our ongoing studies.
The results of our study must be interpreted in light of its limitations. fMRI does not directly measure brain dopamine signaling. However, the well-studied behavior of dopaminergic neurons that is modeled in our computational analysis suggests altered dopamine-related reward processing in the brain in adolescent anorexia nervosa. The mechanism for the elevated prediction error response is uncertain and requires further study, but it may occur through up-regulation of dopamine D
1 or D
2 receptor function (
38). However, nondopaminergic neurons also play a role (
33). Another potential limitation was the group difference in the time between scans. Variability in anorexia nervosa treatment duration is a challenge in studying this patient population, and treatment duration in the anorexia nervosa group did not exactly match the requirement of two menstrual cycles between scans to study healthy controls at a low estrogen state. However, a significant increase in mean BMI was still achieved in the anorexia nervosa group in this period. Although another limitation is that our patient sample used various medications and had various comorbid disorders, this group reflects a typical clinical sample, and we did account for these variables in our analyses. Our main findings held when the one patient with binge/purge type anorexia nervosa was excluded from the analyses (see Table S2 in the
data supplement) and when individuals taking antipsychotics were excluded (see Table S3 in the
data supplement).
In summary, this study suggests that reward learning in general, and independently of primary taste reward, is important for our overall understanding of the neurobiology of adolescent anorexia nervosa. Generalized sensitization of brain reward responsiveness could be a result of food restriction and may last long into recovery, consistent with basic research (
16,
25). Whether individuals with anorexia nervosa have a genetic predisposition for such a sensitization requires further study. Furthermore, our results suggest that elevated prediction error response in the caudate is a marker for illness severity and predicts weight gain in a highly structured treatment program. The mechanism for such a relationship is unclear. However, starvation-induced altered dopamine receptor expression could cause such a phenomenon (
39). Alternatively, metabolic dopamine-related factors could be involved, or high prediction error may characterize a cognitively severe form of adolescent anorexia nervosa that is more “resistant” to treatment. Future studies should aim to elucidate these mechanisms and how elevated dopamine brain response even after weight restoration could be a risk factor for relapse, which is common in the disorder. The answer would have important implications for treatment development. Specifically, reducing high dopamine-related brain response could be a valuable treatment target (
13). To explore this relationship, future studies should include longer-term follow-up measures. Behaviorally, sensitivity to punishment could be related to relapse, as anorexia nervosa behaviors are often described as “safe and predictable.” Whether our above-discussed hypothesis that heightened dopamine-related response triggers high sensitivity to punishment, and that high harm avoidance becomes an adaptive behavior that persists even after weight normalization, remains to be further tested. A comprehensive understanding of the role of other neurotransmitters, such as serotonin, in these mechanisms is also needed (
40). Despite those limitations and the need for replication, this study provides hope that there are biological markers for adolescent anorexia nervosa that could be used in estimating treatment success as well as in developing pharmacological interventions.