Posttraumatic stress disorder (PTSD) is a prevalent condition (
1) with a large burden of suffering (
2). Effective treatments have been developed, the most widely utilized of which are trauma-focused psychotherapies such as prolonged exposure (
3). Although psychotherapy is widely utilized and highly effective, it requires a considerable investment of time and effort, with roughly one-quarter of patients not completing treatment and one-third to one-half of those who complete treatment remaining symptomatic and impaired (
4). It is therefore critical to identify who will benefit from this treatment and why—information that remains largely unknown. Noting that clinical and demographic characteristics are poor predictors (
5), we suggest that brain-based characteristics may serve as particularly robust indicators of treatment outcome.
Data on how brain function prior to treatment predicts psychotherapy outcome in PTSD are sparse. Moreover, past studies offer limited insights or generalizability because of a lack of a patient waiting list condition or a control intervention arm (
6), use of an uncommon treatment modality (
7), or use of small samples (
8). Critically, to our knowledge, there have been no reports of a comprehensive, multifaceted assessment of brain function in a single study; instead, data have been presented separately, from individual paradigms in partially overlapping participant groups.
Here, in a sample that is large relative to published fMRI treatment studies, we identified brain activation that moderates the relationship between treatment arm and symptom change in a randomized clinical trial of prolonged exposure for PTSD. We utilized a patient waiting list comparison group and examined multiple functional tasks united under a common conceptual theme—emotional reactivity and regulation, that is, how an individual recognizes an emotionally charged stimulus, processes that information, and resolves the emotional response. We investigated these processes under the assumption that appropriate reactivity to and regulation of emotion is essential for successful exposure therapy (
9). This is consistent with emotional processing theory, the foundation of prolonged exposure, which holds that confronting feared stimuli to activate the fear response is needed to incorporate information that is incompatible with its pathological structure (
10). This process promotes adaptive learning, which leads to a regulation of fear. Therefore, it is likely that the brain mechanisms that optimize balance between these processes must be intact for the patient to benefit from exposure treatment. Unfortunately, previous treatment imaging studies have typically analyzed data only in participants who complete treatment, which can fundamentally bias results (
11). Here, we thus also adopted a full intent-to-treat analysis framework using linear mixed models, thereby incorporating all available imaging data.
Previous PTSD imaging studies examining predictors of psychotherapy treatment response have reported the following, all in the context of single-arm treatment studies. First, greater activation in the ventral anterior cingulate cortex and the medial prefrontal cortex during nonconscious fear processing was found to predict poorer response to cognitive-behavioral therapy (CBT) (
6). Second, greater activation in the dorsal anterior cingulate during nonconscious fear processing (
6) and anticipation of negative versus positive emotional images predicted better CBT response (
12), but less dorsal anterior cingulate activation during image presentation also predicted better CBT response (
12,
13). Third, less amygdala reactivity to nonconscious fear processing (
6) and conscious processing of negative pictures (
13) predicted a better response to CBT.
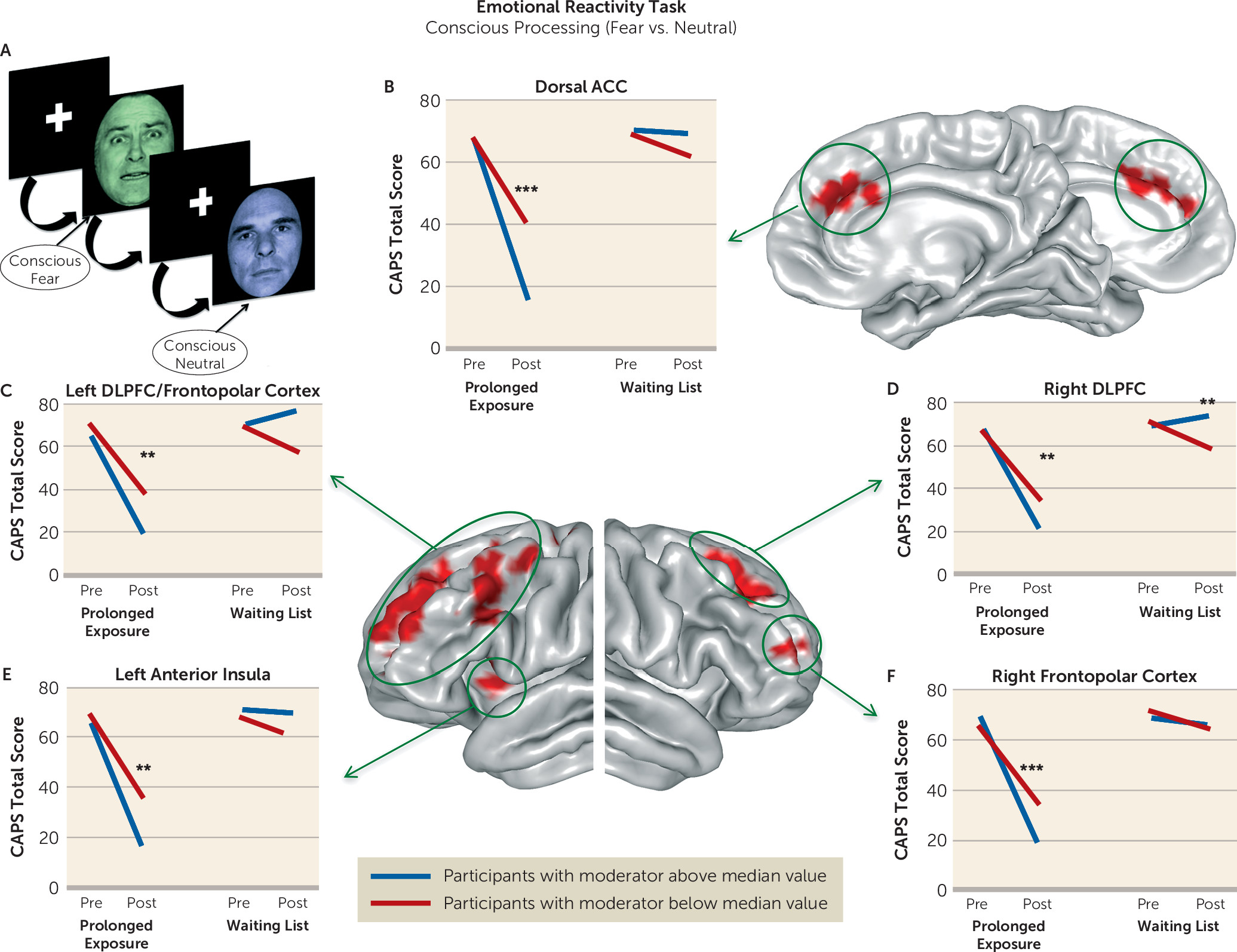
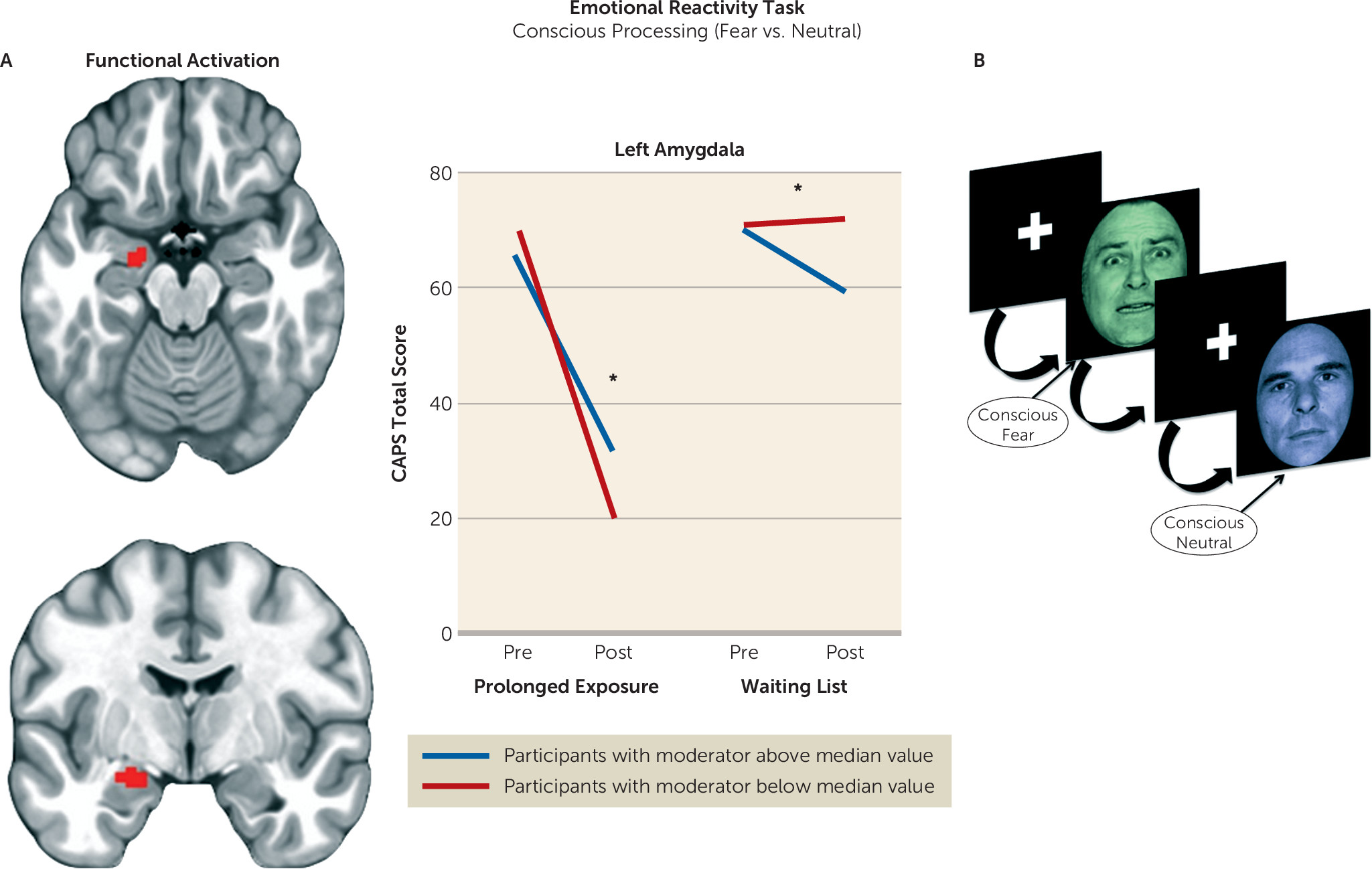
Therefore, we formulated the following hypotheses. First, we expected individuals with less amygdala activation during emotion detection at baseline to show a greater reduction in symptom scores after treatment. Second, we expected individuals with less ventromedial prefrontal activation during nonconscious fear processing to show a greater reduction in symptom scores after treatment (
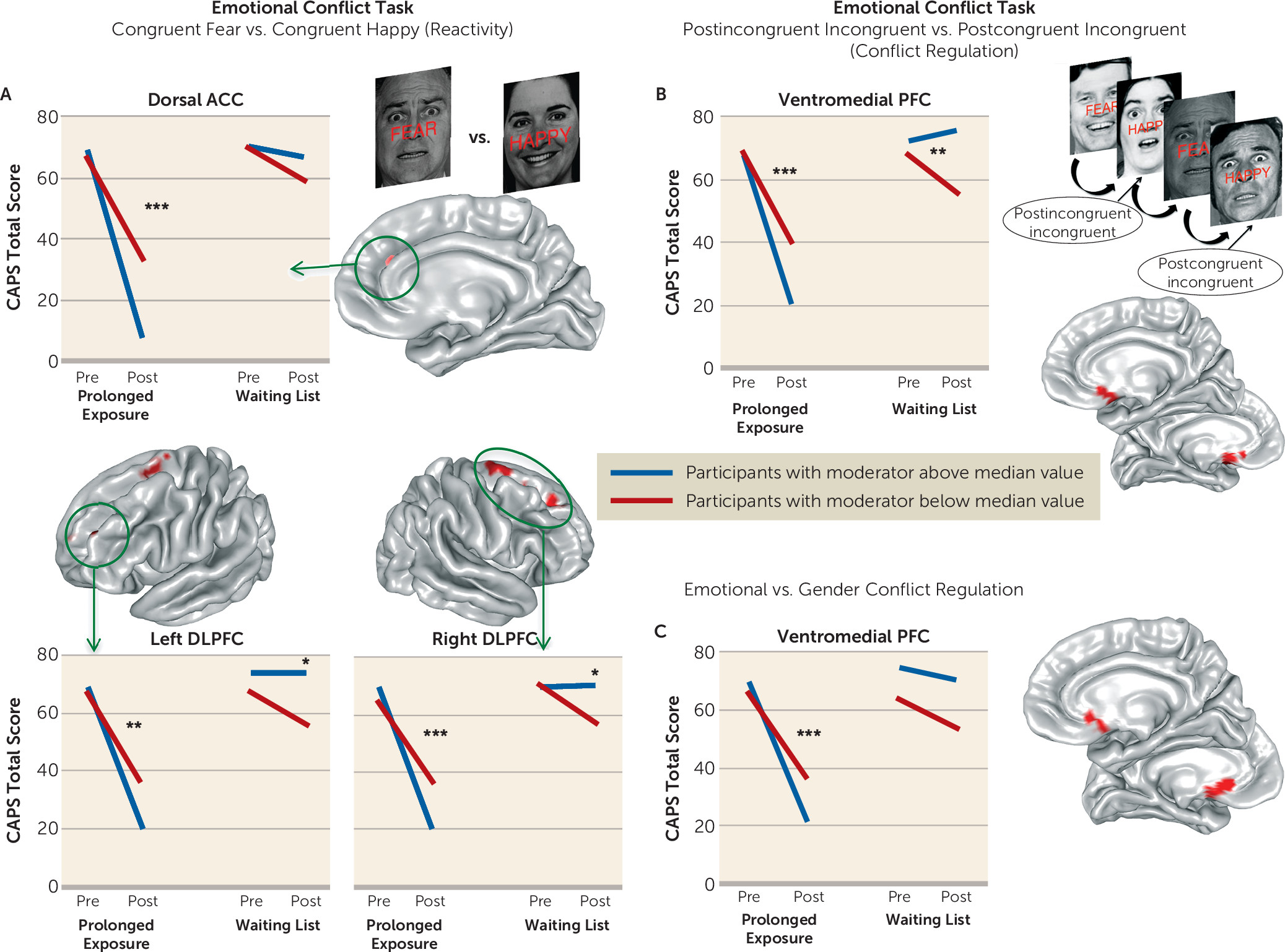
6). However, we also expected individuals with greater ventromedial prefrontal activation during emotional conflict regulation to show a greater treatment-related reduction in symptom scores. This is consistent with previous work implicating this region in emotional conflict regulation (
14) and fear extinction (
15), which we anticipated would support exposure habituation and improve treatment efficacy. Third, we predicted that activation of the rostral/dorsal anterior cingulate when processing an emotional cue would moderate the relationship between treatment arm and symptom change, consistent with previous work, although we did not have an a priori directional hypothesis, given inconsistent previous findings (
6,
12). Finally, we hypothesized that individuals with greater activation of dorsolateral prefrontal regions during processing and deliberate regulation of negative emotion would demonstrate greater treatment-related reductions in symptom scores, given the role of these regions in emotion regulation and their importance in existing models of psychotherapy mechanisms (
16).
Discussion
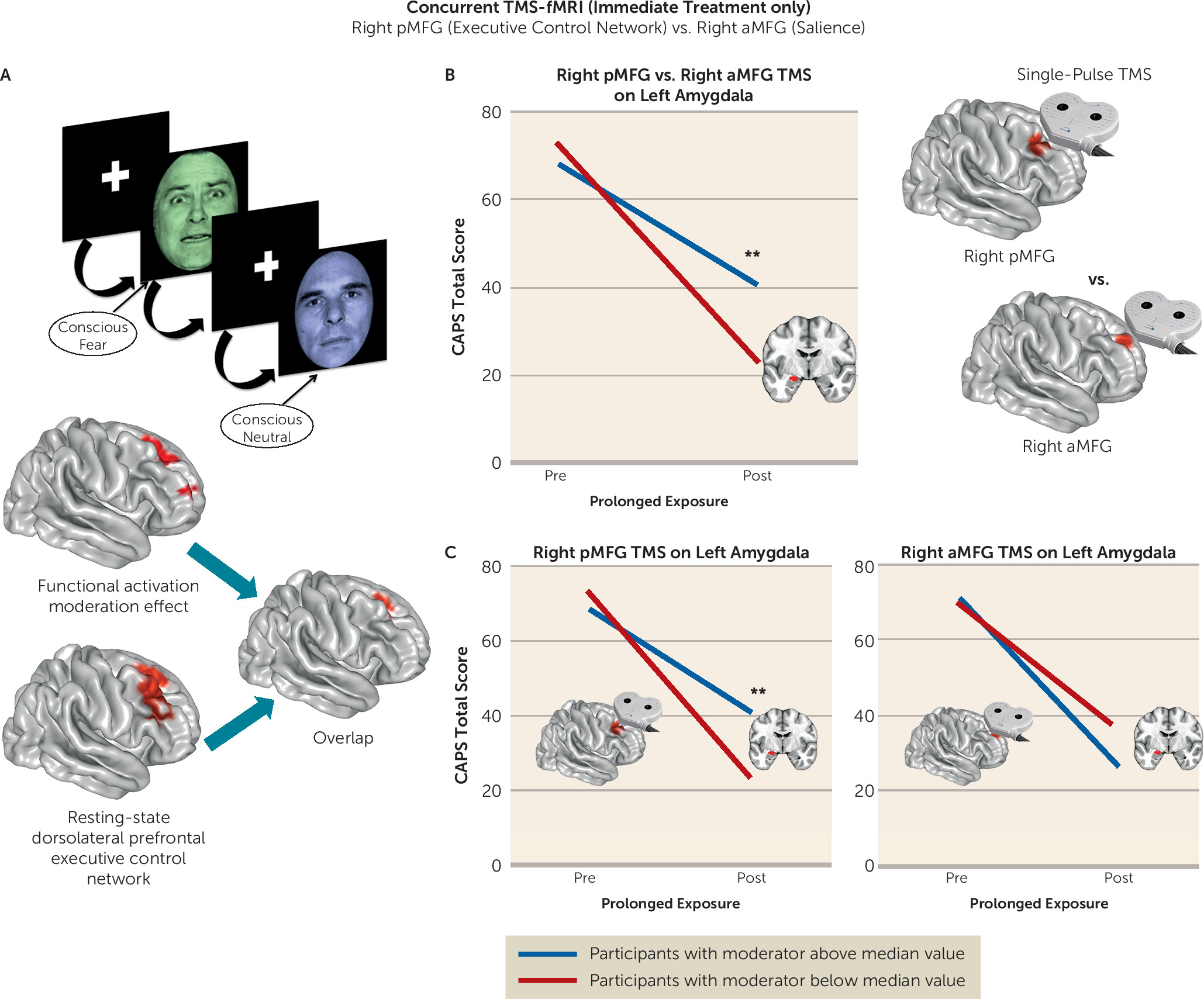
We undertook a rigorous investigation of the functional brain characteristics during emotional reactivity and regulation that moderate differential symptom change from prolonged exposure therapy compared with a waiting list condition in PTSD. We also incorporated causality-focused TMS-fMRI manipulations to enhance the interpretability of the task findings. The primary results are as follows. First, individuals with greater baseline recruitment of the dorsal anterior cingulate, anterior insula, and dorsolateral prefrontal cortex as well as less amygdala activation when incidentally processing an emotional stimulus showed larger reductions in symptom scores after treatment. TMS-fMRI findings recapitulated this dynamic, demonstrating that the magnitude of downstream inhibition of the left amygdala from right dorsolateral prefrontal stimulation moderated the effect of treatment on symptoms. Second, individuals with greater baseline ventromedial prefrontal/ventral striatal activation during implicit regulation of emotional conflict demonstrated larger symptom reductions after treatment. Notably, this effect was specific for regulation of emotional (as opposed to nonemotional) content. Thus, an individual’s capacity to benefit from exposure therapy is gated by 1) degree of spontaneous prefrontal control over amygdalar threat detection signals during incidental processing of a fear-conveying stimulus and 2) the brain’s capacity to reduce interference from an emotional cue in the environment.
Interestingly, brain activation during deliberate regulation of one’s emotional state did not predict treatment-related symptom change. This observation dovetails with clinical research, which emphasizes emotional engagement during an exposure while refraining from deliberate attempts to attenuate emotional responses (
24,
29). The capability for this type of emotional engagement may actually depend on one’s capacity to devote attention to both the emotional experience itself and other simultaneous aspects of one’s experience (such as goals and intentions). We believe this capacity is engaged by the emotional reactivity task used here, which induces a goal orientation (color tint identification) concurrent with the emotional stimulus. Prefrontal engagement during this process may be indicative of greater top-down resources devoted to the appraisal of the emotional stimulus and modulation of attention toward nonemotional components (
30), perhaps indexing an individual’s capability to attend to goal-relevant processes in the presence of perceived environmental threat, for example, sustaining an exposure exercise in the presence of fear. This is consistent with the roles of the dorsal anterior cingulate in appraisal of fear (
31) and the dorsolateral prefrontal cortex in top-down attentional control (
30). Thus, this uninstructed individual tendency toward engaging greater prefrontal control when appraising an emotional stimulus and modulating attention in relation to it may be a type of “spontaneous” emotion regulation that augurs well for engagement in and therapeutic benefit from exposure therapy. This interpretation is consistent with the results of single-pulse TMS manipulations, which likewise provide a potential causal mechanism for the efficacy of repetitive TMS to the right dorsolateral prefrontal cortex in treating PTSD (
23).
Anterior insula activation during emotional reactivity also moderated the relationship between treatment arm and symptom change. Although this region is involved in processing fear and is known to be hyperactive across anxiety manifestations (
32,
33), it is involved in numerous processes, including attention, working memory, language, and perceptual processing (
34,
35). The insula can be functionally subdivided into a dorsal cognitive region and a ventral emotional subdivision (
34,
36). The effect we detected was located in the more dorsal portion (at z=6 in the cluster center of mass), which is consistent with the role of this more dorsal anterior insular region in attentional allocation (
36). Conversely, emotion-related meta-analytic insular activations tend to be more ventrally located (
34). We therefore interpret this effect to signify greater processing resources being devoted toward allocating attention away from the emotional content of the face and toward the color tint (the focus of the task), consistent with the observed concomitant moderating activation of the dorsal anterior cingulate and dorsolateral prefrontal cortex—regions heavily implicated in attention shifting (
37) and in facilitating attentional control in conjunction with the insula (
38).
Emotional conflict regulation normally recruits the ventromedial prefrontal cortex (
14), is perturbed in individuals with ventromedial prefrontal lesions (
28), is abnormal in some affective disorders (
39), and is thought to index implicit regulation of interference from an irrelevant emotional stimulus (
40). We found that ventromedial prefrontal/ventral striatal recruitment during emotional conflict regulation moderated the relationship between treatment arm and symptom change in an emotion-specific manner. Activation here was also correlated with behavioral indices of emotional conflict regulation at baseline. Localization of this effect to the posterior portion of the ventromedial prefrontal cortex (BA 25, subgenual cingulate) and the adjoining rostroventral striatum may reflect how attunement to goal-relevant emotional information and reduction of perturbation from a salient stimulus results in reduced arousal or vigilance. This is consistent with the positive relationship between subgenual cingulate activation and parasympathetic processes (
41) and the crucial role of nucleus accumbens shell (the rostroventral striatum) in mediating the resistance of the brain to associating a previously encountered harmless stimulus with a salience signal for a future aversive outcome (
42). This is also consistent with translational neuroscience findings that implicate the infralimbic cortex in rats (the ventromedial prefrontal cortex in humans) in facilitating fear extinction (
43), as conflict regulation and fear extinction share some conceptual and empirical overlap (
31). In relation to exposure, we interpret this effect to be a marker of the brain’s capacity to attenuate heightened arousal or vigilance following stimulus-cued fear responses.
It is notable that many of the brain activation moderator effects were predictive of outcomes in both the immediate treatment and waiting list arms in opposite directions. We speculate that these effects reflect regulatory mechanisms that are engaged differently by “long-term” and “short-term” symptom coping techniques. When we refer to “long-term” techniques, we denote therapeutic exercises such as in vivo and imaginal exposure, which promote recovery and lasting adaptive change. By “short-term” coping, we refer to techniques that are readily available and have a lower time and energy cost for the individual, such as active avoidance and distraction. Given the emphasis of the treatment on emotional processing via exposure (
10) and minimization of avoidance, these opposite mechanistic relationships are therefore enforced by the randomization. Although “short-term” coping (the only type available to participants in the waiting list condition) may provide some limited symptom relief, we note that none of the participants in the waiting list condition demonstrated naturalistic recovery from PTSD, and only about half of those who completed the waiting list condition (N=13) showed any decrease in PTSD symptoms. Ultimately, naturalistic recovery would need to be studied in a controlled context without treatment, over a longer period, and in a larger sample for valid inferences to be made.
This study has several limitations. First, we did not examine a trauma-exposed healthy control sample, which may provide insight regarding how compensatory adaptations or pathological markers interact with treatment to guide outcomes. Second, we did not investigate trauma-specific domains, such as symptom provocation, or experimental constructs of proposed etiological pathways, such as fear conditioning/extinction. These are likely to provide useful complementary information. Third, the sample size, although large for a PTSD imaging treatment study, is relatively small for a randomized clinical trial and for examining moderation effects. Therefore, additional studies are needed to replicate and extend these findings and validate their utility for clinical decision making. This is particularly true of the TMS-fMRI findings, to which only a subset of the sample contributed. Fourth, we did not counterbalance task order across participants, as it was not possible to ensure balanced administrations across randomized groups. This could reduce generalizability of brain moderation effects if the order of administration exerted habituation effects on brain dynamics that moderated the effect of treatment on symptoms.
In conclusion, we highlight three primary insights from this study that speak to the importance of targeted brain assessments for identifying individuals with PTSD who are likely to benefit from exposure treatment and how the knowledge derived from these brain assessments can improve clinical outcomes. First, assessing individuals’ brain activation patterns can greatly improve our ability to predict remission from PTSD with treatment, beyond typical clinical and demographic measures. Second, our findings identify neurostimulation-accessible cortical regions that could serve as treatment targets for augmenting brain function prior to or concurrent with psychotherapy, thereby potentially “conditioning” the brain to respond to therapy. Third, these findings highlight the relevant behavioral constructs likely to provide a useful predictive signal of an individual’s response to exposure therapy. Further development of techniques to assess these predictive brain markers with clinic-friendly measurement tools, for example, electroencephalography, will allow for the determination of an individual’s suitability for prolonged exposure in a clinic setting. The present findings thus inform future efforts at individualized treatment selection and provide much-needed mechanistic insights regarding the neural phenotypes that respond best to exposure therapy.