Apathy, a profound loss of motivation, is the most common behavioral problem in Alzheimer’s disease (
1). Apathy is characterized by indifference, disengagement, passivity, and loss of enthusiasm, interest, and empathy. A recent review of behavioral and psychological symptoms of dementia found that apathy was the only one among eleven symptoms with high baseline prevalence, persistence, and incidence over the course of dementia (
2). In a population-based study of adults (N=3,626) ages ≥65 years, apathy was one of the most persistent symptoms noted during 10-year dementia follow-up (
3). Those with apathy had a 62% probability of remaining apathetic after 1 year. Hence, apathy clearly persists if left untreated.
Apathy has profound consequences for both patients and caregivers. The presence of apathy with Alzheimer’s disease is associated with functional impairment. Even after controlling for age, education, cognition, and depression (
4,
5), those with apathy are 1.9–2.8 times as likely to have deficits in activities of daily living compared with those without apathy (
6,
7). In fact, apathy and other behavioral problems may have more impact on everyday function than cognition (
8). Apathetic patients require more management and support, leading to increased caregiver burden and service utilization. Even more alarming is the fact that apathy is associated with higher mortality. In a population-based study, the presence of apathy was linked with 3.1 times higher mortality than those without apathy (
3). Hence, it is important to treat apathy. Because apathy affects function, illness trajectory, and mortality, treating it in early dementia while the patient is dwelling in the community could have substantial benefits.
Unfortunately, pharmacological treatment options for apathy are limited. Medications currently approved for Alzheimer’s disease have had mixed results in treating apathy. Cholinesterase inhibitors were found to be effective in improving apathy in secondary analyses, but memantine was not (
9,
10). Our team and others have found modest improvements in apathy and cognitive correlates with dopaminergic agents (
10–
15). However, only two of these studies were randomized controlled trials (
16). Although of relatively short duration (2 and 6 weeks), both reported favorable results with use of methylphenidate (
11,
14). A crossover randomized controlled trial in moderate Alzheimer’s disease (N=13) found that 2 weeks of methylphenidate treatment significantly improved Apathy Evaluation Scale–Informant (AES-I) scores but worsened Neuropsychiatric Inventory apathy scores compared with placebo (
11). The other randomized controlled trial in moderate Alzheimer’s disease (N=60) found that, compared with placebo, 6 weeks of methylphenidate treatment resulted in significant improvement in the Neuropsychiatric Inventory apathy scores but not in the AES-I scores (
14). Both studies demonstrated early effects of methylphenidate and concluded that longer treatment may lead to better response.
The primary objective of the present study was to test the effects of 12 weeks of methylphenidate treatment on apathy in community-dwelling older veterans with mild Alzheimer’s disease. The secondary objectives were to explore the effects of methylphenidate on cognition, functional status, caregiver burden, Clinical Global Impressions Scale (CGI) scores (improvement [CGI-I] and severity [CGI-S]), and depression.
Method
Study Design and Participation
A 12-week, double-blind, randomized, placebo-controlled trial was conducted at a Department of Veterans Affairs medical center to compare the effects of methylphenidate with placebo on apathy in community-dwelling older veterans with Alzheimer’s disease. The protocol was approved by the institutional review board. Participants were recruited via advertisements, presentations to professional and caregiver support groups, provider referral, and patient records search. After appropriate approvals were obtained, a list was generated of all patients seen over the previous 6 years with various dementia diagnoses, living within a zip code, and excluding those with glaucoma and those currently using antipsychotics and stimulants. A total of 979 names were obtained. Letters were sent out in batches of 100 with a number to opt out of telephone calls. Four hundred letters were sent prior to reaching the recruitment goal. Phone calls were made approximately 2 weeks after the letters were mailed. Most initial telephone conversations were with the caregiver. If interested after the study description, caregivers indicated whether the patient had a diagnosis of dementia and issues with loss of motivation. If both problems were affirmed, permission was requested to further prescreen the patient’s medical records. Eligible patients and their caregivers were invited for a baseline visit.
Inclusion and Exclusion Criteria
After assessment of capacity to consent and provision of written informed consent, the diagnosis of Alzheimer’s dementia was confirmed by the principal investigator (P.R.P., a board-certified geriatric psychiatrist) using clinical history and DSM-IV criteria. Further screening occurred by medical history, physical examination, and tests for apathy and cognition. Alzheimer’s disease patients were included who had a caregiver; scored ≥18 on the Mini-Mental State Examination (MMSE) and >40 on the Apathy Evaluation Scale–Clinician version (AES-C); and, if applicable, were taking stable doses of antidepressants for at least 2 months or taking cholinesterase inhibitors or memantine for at least 4 months. Patients were excluded if they were taking methylphenidate or if they had prior adverse events with methylphenidate; if they were taking other exclusionary medications (amphetamines, antipsychotics, monoamine oxidase inhibitors, or clonidine); if they had frontotemporal dementia, current major depressive disorder, or active psychosis, as determined by the Mini International Neuropsychiatric Interview (MINI) (
17); or if they had a history of uncontrolled seizures, uncontrolled hypertension, symptomatic cardiovascular disease or cardiomyopathy, Tourette’s syndrome, or closed-angle glaucoma.
Study Intervention
After baseline data collection (demographic, anthropometric, outcome measures), participants were randomized to methylphenidate (N=30) or placebo (N=30) groups using a random block design developed by a statistician using sealed envelopes. The participants, study team, and outcome assessors were masked to treatment assignment. Study medication was counted and dispensed by the research pharmacy. All participants were started on 5 mg of methylphenidate (or look-alike placebo) twice daily and titrated to 10 mg twice daily at 2 weeks. The first dose was taken early in the morning and the second dose no later than 3 p.m. to avoid sleep disturbance. Participants were instructed not to make up missed doses. The protocol allowed the study physician to decrease the dose if adverse events were reported. Otherwise, participants continued to take 10 mg twice daily until 12 weeks, at which time the dose was tapered to 5 mg twice daily for 3 days and stopped.
Outcome Measures
The primary outcome measure was score on the AES-C. The secondary outcome measures were scores on the Modified Mini-Mental State Examination (3MS) and on the MMSE; instrumental activities of daily living; activities of daily living; scores on the Zarit Burden Scale, the CGI-I and CGI-S scales, and the Cornell Scale for Depression in Dementia (dementia depression scale); and adverse events. Assessments were conducted at the medical center by a trained study team blind to whether drug or placebo was administered. Apathy scale ratings were performed by clinicians, and staff performed the remaining ratings. Interrater reliability was confirmed periodically. The AES-C was measured at baseline and at 4, 8, and 12 weeks. The 3MS, MMSE, CGI-I, CGI-S, weight, and vital signs were measured at baseline and at 4 and 12 weeks, while activities of daily living, instrumental activities of daily living, the Zarit Burden Scale, and the dementia depression scale were measured at baseline and at 8 and 12 weeks. Electrocardiogram tests were obtained at baseline and at 4 and 8 weeks.
Apathy
The AES-C is an 18-item scale that measures behavioral, cognitive, and emotional domains of apathy in the previous 4 weeks. A clinician gathers information from both the patient and caregiver using a semistructured interview. Scores range from 18 to 72, with 30 or higher generally considered clinically significant. A cutoff of 40 has been suggested in patients with dementia (
18). The AES-C has good internal consistency (Cronbach’s alpha=0.94) and retest reliability (r=0.94) (
19). Although clear data on clinically meaningful difference are unavailable, a change score of 3.3 is generally considered a meaningful improvement (
12). Domains of apathy are calculated by summating scores of pertinent items (
19).
Cognition
The 3MS is a screen for global cognition that assesses orientation, registration, recall, simple language, and construction. Retest reliability ranges from 0.91 to 0.93 (
20). The scores range from 30 to 100 and are superior to the MMSE as a screen for dementia (
21). Different versions were used at each visit to minimize practice effects. An MMSE score derived from the 3MS was used for study inclusion (
20).
Function
The instrumental activities of daily living scale assesses eight domains of higher functions necessary to live independently, such as cooking, shopping, and managing medications and finances. Scores range from 0 to 23, with higher scores indicating better function (
22). The activities of daily living scale assesses independence in performing basic tasks, such as feeding, dressing, and bathing. Scores range from 0 to 24, with higher scores indicating better function (
23).
Caregiver Burden
The Zarit Burden Scale is a widely used 22-item assessment of the caregiver’s burden related to patient relationship, general medical and mental health, finances, and social life. Caregivers rate items from 0 (never) to 4 (nearly always), and higher scores indicate higher burden. This scale has excellent reliability (
24).
CGI
The CGI is an observational global evaluation that assesses the change in degree of illness relative to an original assessment (
25). Two components are evaluated, overall clinical severity (CGI-S) and improvement (CGI-I). At intake, CGI-S scores were rated, as only severity can be rated. In subsequent assessments, both severity and improvement were rated.
Depression
The Cornell Scale for Depression in Dementia is validated for quantifying depression in dementia. It has 19 items, each rated as 0 (absent), 1 (mild or intermittent), or 2 (severe). A score of ≥9 indicates depression in dementia (
26). In a review of more than 3,000 elderly outpatients with dementia, this scale had a sensitivity of 0.84 and a specificity of 0.80 (
27).
Adverse Event Monitoring
Data on adverse events were collected at in-person and telephone visits. Known adverse events associated with methylphenidate listed in the package insert were tabulated, and open-ended questions were asked to identify unexpected adverse events.
Statistical Analyses
Demographics and baseline characteristics were compared using t tests or nonparametric Wilcoxon rank sum tests (continuous data) and Fisher’s exact test (categorical data). Changes from baseline at 4, 8, and 12 weeks in primary and secondary outcomes, as well as apathy domains (cognitive, behavioral, emotional, motivation, persistence, and novelty), were analyzed using repeated-measures mixed model analyses of covariance. Group (methylphenidate and placebo) and time (4, 8, and 12 weeks), as well as the interaction between the two, were independent variables; the dependent variable’s baseline measure was included as a covariate. In post hoc analyses, groups were compared in terms of change from baseline at 4, 8, and 12 weeks using t tests derived from model-based contrasts. Additional comparisons were made to detect within-group improvements. An interaction term was added to the model to evaluate how depression (the dementia depression scale), antidepressants, and cholinesterase inhibitors at baseline modified the treatment effect. In addition, an interaction term was added to the model to evaluate how the history of depression at baseline modifies the treatment effect. Two-sided p values less than 0.05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide, version 5.1 (SAS Institute, Cary, N.C.).
Results
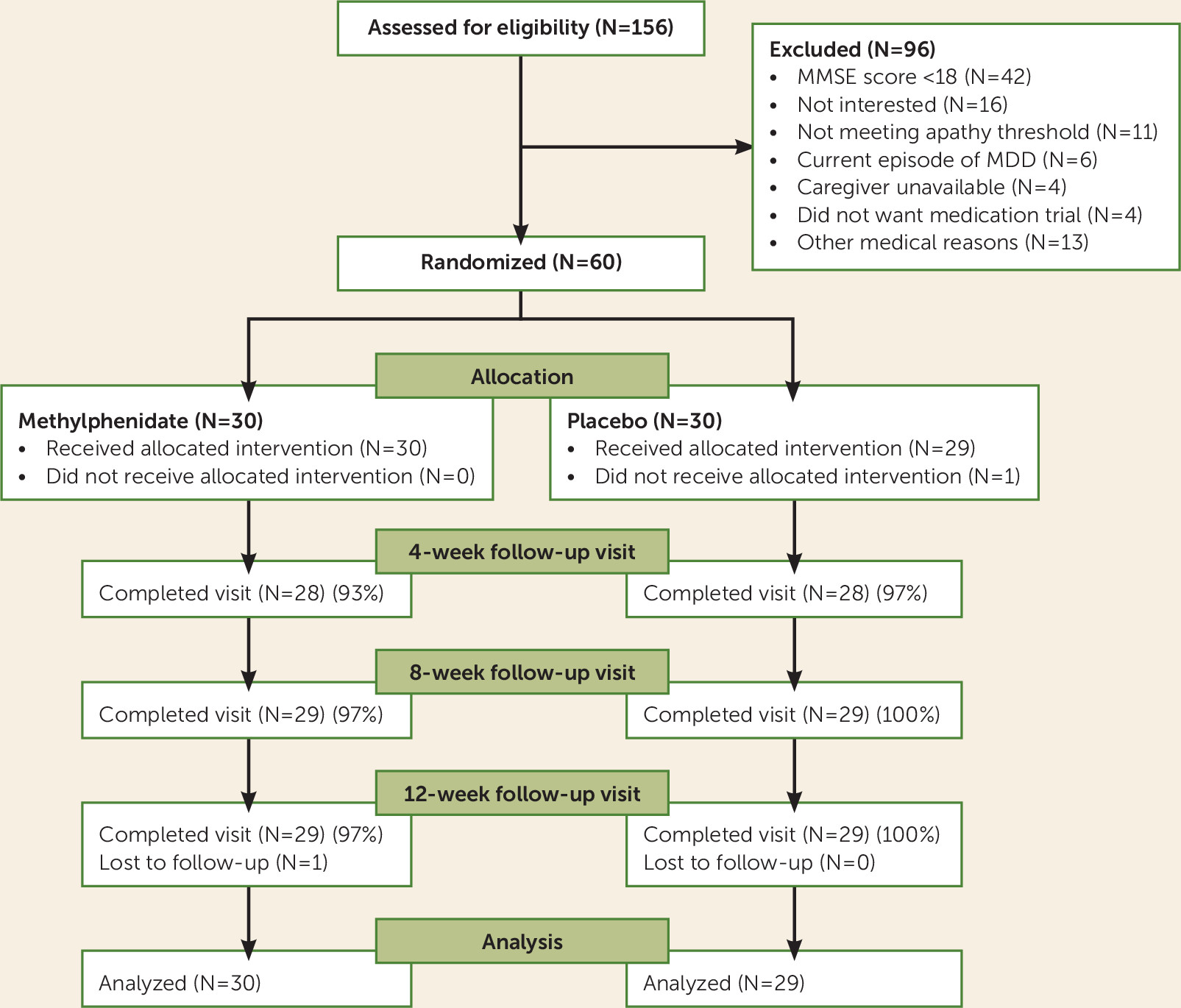
Screening, enrollment, and participation information is shown in
Figure 1. Following review of recruitment records (N=400) and in-person screening (N=156), eligible subjects (N=60) were randomized to the methylphenidate or placebo groups. One subject withdrew from each arm due to caregiver unavailability. Most participants attended all visits (
Figure 1). The study demographics are presented in
Table 1. All participants were men, which reflects the veteran population age ≥60 years. There were no significant differences between groups with regard to age, race, gender, concomitant medications, or comorbidities, except coronary artery disease (
Table 1). Depression was common (58%). No participant met criteria for a current episode of major depressive disorder per the MINI. Concomitant medications and comorbidities are listed in
Table 1. There were statistically significant between-group differences at baseline in the AES-C, the activities of daily living scale, the instrumental activities of daily living scale, and the CGI-S. The methylphenidate group had higher apathy scores, lower functional status, and higher CGI-S scores than the placebo group (
Table 2). The target dosage (10 mg b.i.d.) was achieved on all participants in the methylphenidate group except in three individuals, where the dosage was reduced to 5 mg b.i.d. because of elevated blood pressure, hospitalization for seizure, and insomnia. The mean final dosage of methylphenidate was 9.5 mg b.i.d.
Primary Outcome: Apathy
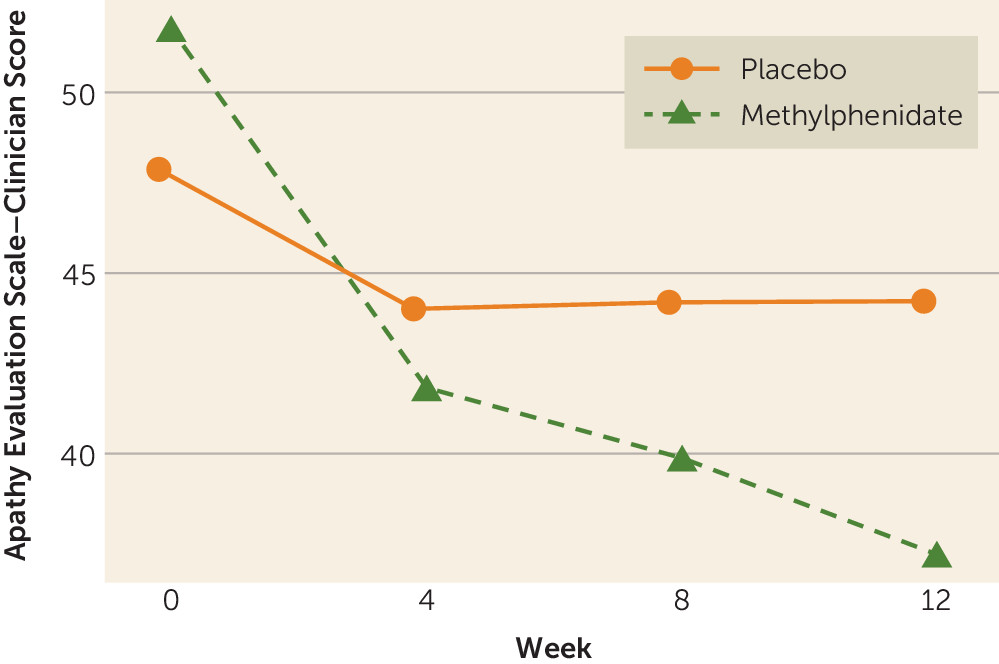
There was a significant group-by-time interaction for the primary outcome measure, scores on the AES-C (p<0.016), in those completing the study (N=58) (
Figure 2). There were significant differences over time for apathy in the methylphenidate group (p<0.001) but not in the placebo group (p=0.983). After adjusting for baseline scores on the AES-C, we found that the methylphenidate group had significantly greater improvement than the placebo group (average between-group differences) at 4 weeks (−5.2, 95% CI=−9.0 to −1.5, p=0.006), at 8 weeks (−7.2, 95% CI=−10.9 to −3.5, p<0.001), and at 12 weeks (−9.9, 95% CI=−13.6 to −6.2, p<0.001) (
Table 3). There was significant within-group improvement in scores on the AES-C in both groups at all time points; however, the change in the methylphenidate group was significantly higher than that in the placebo group.
Apathy Domains
Improvement seen in scores on the AES-C in the methylphenidate group was driven by improvements in multiple apathy domains. The behavioral domain had greater improvement in the methylphenidate group compared with the placebo group at 8 weeks (−2.4, 95% CI=−3.8 to −1.0, p<0.001) and at 12 weeks (−2.6, 95% CI=−4.0 to −1.2, p<0.001). Similarly, the cognitive domain showed greater improvement in the methylphenidate group compared with the placebo group at 8 weeks (−1.9, 95% CI=−3.9 to 0.0, p=0.050) and at 12 weeks (−3.6, 95% CI=−5.5 to −1.6, p<0.001). Improvement in the emotional domain favoring the methylphenidate group reached statistical significance only at 12 weeks (−1.1, 95% CI=−1.8 to −0.4, p=0.003). The motivation domain improved significantly in the methylphenidate group compared with the placebo group at 8 weeks (−1.3, 95% CI=−2.2 to −0.4, p=0.009) and at 12 weeks (−1.6, 95% CI=−2.5 to −0.6, p=0.001). Greater but nonsignificant improvement in the methylphenidate group was found for the novelty (p=0.052) and persistence domains (p=0.053) only at 12 weeks.
Secondary Outcome Measures
The methylphenidate group had significantly greater improvement in cognition—3MS (6.1, 95% CI=2.7–9.6, p=0.001) and MMSE (2.6, 95% CI=1.1–4.0, p=0.001) scores—than the placebo group at 12 weeks (
Table 3). Significant between-group differences favored the methylphenidate group at 12 weeks for measures on the scale of instrumental activities of daily living (2.3, 95% CI=0.7–3.9, p=0.005), on the Zarit Burden Scale (−5.8, 95% CI=−10.1 to −1.4, p=0.011), on the CGI-I (−1.3, 95% CI=−1.9 to −0.6, p≤0.001), on the CGI-S (−1.1, 95% CI=−1.6 to −0.6, p<0.001), and on the dementia depression scale (−2.5, 95% CI=−4.2 to −0.8, p=0.004) (
Table 3). CGI-I scores also showed significant between-group difference at 4 weeks (
Table 3). There were no between-group differences in weight, pulse, systolic blood pressure, and diastolic blood pressure at 4 weeks or at 12 weeks. However, there was a significant within-group increase in systolic blood pressure in the methylphenidate group at 12 weeks (p<0.001). There was also a within-group decrease in pulse in the placebo group at 4 weeks (p=0.063), which reached statistical significance at 12 weeks (p=0.006) (
Table 3).
In addition, the three-way interaction among dementia depression scale scores and use of antidepressants and cholinesterase inhibitors at baseline with regard to week and treatment group was not significant (analyzed in separate models), indicating that the treatment effect over time did not significantly differ according to these covariates. None of the two-way interactions (covariate by group) were significant (p=0.231, 0.133, and 0.910, respectively). However, for higher scores on the dementia depression scale, the treatment effect was more pronounced even though the effect fell short of significance. For example, at 12 weeks, the estimated treatment effect (95% CI) was −11.3 at a dementia depression scale score of 8 (highest quartile), compared with −7.0 at a dementia depression scale score of 4 (lowest quartile). Similarly, the treatment effect over time did not significantly differ according to the presence of baseline depression.
Adverse Events
Twenty-two participants experienced adverse events (in the methylphenidate group, N=13; in the placebo group, N=9) (
Table 4). There were no deaths, although serious adverse events (N=6) and nonserious adverse events (N=22) occurred. Serious events were related to hospitalizations, although only one was possibly or probably related to study participation (seizure, methylphenidate arm). The most common nonserious events were dizziness and insomnia (N=5 each). Participants (N=2) in the placebo arm experienced severe insomnia. The other nonserious adverse events were of mild to moderate intensity. There were no significant differences in the proportion of participants between groups experiencing adverse events (p=0.284) or serious adverse events (p=0.195).
Discussion
The main objective of the study was to compare the effects of methylphenidate with placebo on apathy in community-dwelling older veterans with mild Alzheimer’s disease. There was significantly greater improvement in apathy with methylphenidate compared with placebo after 4 weeks of treatment, and apathy continued to improve with methylphenidate at 8 and 12 weeks, with the highest between-group difference at 12 weeks. The behavioral and cognitive domains improved by 8 weeks, and the emotional domain finally improved at 12 weeks. These results suggest that improvement in the emotional domain may be mediated by improvements in the cognitive and behavioral domains. Similarly, the motivation domain improved as early as 8 weeks, while the persistence and novelty domains did not reach statistical significance even at 12 weeks. Distinct brain circuits may play a role in different domains of apathy (
28). Cognitive and behavioral apathy is linked to anterior cingulate dysfunction, and emotional apathy to the anterolateral frontal cortex (
29,
30). Although it is not known if methylphenidate acts differentially on dopaminergic input in these areas, specific neuromodulation techniques could be developed to personalize the treatment of apathy.
The methylphenidate group also experienced significantly greater improvement than the placebo group in cognition, functional status, caregiver burden, and depression, although effects were not seen until 12 weeks. The 2.6-point change in MMSE scores at week 12 is comparable to that seen with cholinesterase inhibitors (
31).
The results of our study are consistent with the previous two randomized controlled trials of methylphenidate for apathy in Alzheimer’s disease (
14). These three randomized controlled trials collectively support the dopaminergic hypothesis of apathy. Although the ideal duration of treatment with methylphenidate is unknown, a case for longer duration can be made because the results of these studies were proportional to the duration of treatment, with the highest improvement in apathy and cognition noted with 12-week treatment. Longer duration studies need to be conducted to investigate if the improvement in apathy continues with the duration of treatment or if it plateaus at a certain point.
Several other study factors could have contributed to the robust improvement in apathy and cognition. Participants in the present study were community dwellers and had mild dementia, suggesting that methylphenidate could have had more robust effects when used earlier in the trajectory of neurodegeneration. Another unique feature of our study that may have influenced the results is the gender of the participants. It is recognized that apathy increases with age and much more so in men, possibly from effects of declining testosterone levels (
32). Furthermore, gender differences are noted in symptom response to methylphenidate treatment in children with attention deficit hyperactivity disorder, with better response in males (
33,
34). Thus, apathy may improve preferentially among men in response to methylphenidate.
Other differences among the aforementioned three methylphenidate randomized controlled trials may explain the different outcomes. Our study did not include a standardized psychosocial intervention as was done in one of the previous randomized controlled trials (
14). The primary outcome measure in our study was the AES-C, whereas the AES-I was used in prior studies (
14). The AES-I may not discriminate apathy from depression as well as the AES-C, and thus, changes in the AES-I could have been confounded by changes in depression scores (
19). Furthermore, the variation in the scoring of the AES-I might be influenced more by an interest factor than the apathy factor, which is the main source of variance in the AES-C (
18).
A lag in improvement in cognitive and functional outcomes, relative to apathy measures, was noted. The scale of activities of daily living did not change significantly because most participants were high functioning, as expected in a community-dwelling cohort. Caregiver burden improved between groups only at the 12-week mark, suggesting that this result may be a distal outcome influenced by the apathy, cognitive, and functional outcomes. Although the cohort on average was nondepressed according to dementia depression scale cutoffs at baseline, there was improvement in depressive symptoms by 12 weeks. The change in depression scores is similar to what is known with methylphenidate, but the response was delayed (
35). Neither the baseline history of depression nor the treatment with antidepressants influenced apathy outcomes, but higher baseline scores on the dementia depression scale were associated with greater treatment effect. This finding illustrates that although there is some overlap between apathy and depression, apathy is a distinct entity warranting additional treatment. Similarly, the treatment effect over time did not significantly differ according to cholinesterase inhibitor treatment at baseline. This finding hints at additional benefit that stimulants could offer for apathy, over and above the effect of cholinesterase inhibitors. Weight loss, changes in heart rate and blood pressure, and insomnia are common side effects of methylphenidate that prevent its use. In the present study, there were no between-group differences in these measures. However, those receiving methylphenidate were noted to have statistically and perhaps clinically significant change in systolic blood pressure (median increase of 7 mmHg). This finding agrees with other published reports (
36). In our study, the baseline systolic blood pressure and change in systolic blood pressure did not vary per group. Although there is no evidence of a statistical interaction, it is possible that the presence of hypertension at baseline does make it more likely for increased systolic blood pressure at follow-up due to the sympathomimetic action of methylphenidate.
The major strength of the study is its design: a double-blind, randomized, placebo-controlled trial in community-dwelling older veterans with mild Alzheimer’s disease. Other strengths include that the medications were well tolerated and that there were few dropouts (N=2). However, the study has several limitations. This study was conducted in a select group (veterans), and all participants were men. The study was also conducted at a single site and had a small sample size. The study also lacked biomarkers.
In conclusion, 12-week methylphenidate treatment improved apathy in community-dwelling older veterans with mild Alzheimer’s disease. Methylphenidate also improved cognition, functional status, caregiver burden, CGI scores (improvement and severity), and depression.