Early-life stress is thought to cause many adverse health outcomes, including altered brain development (
1), compromised cognitive functioning (
2), poor mental health (
3), and multiple physical illnesses (
4). Perhaps the most pressing question at the nexus of neurobiology and public health is how stress “gets under the skin” to bring about these pleiotropic effects (
5–
9). Although the molecular mechanisms linking early-life exposures to these multiple outcomes are not yet understood, vigorous scientific attention is currently focused on the role of long-term alterations to gene expression and function, mediated by dynamic epigenetic modifications (
10). In particular, DNA methylation has been proposed as a mechanism by which early-life experiences may become “embedded” in the genome (
11), and this possibility has captured the public imagination as a means by which stress becomes toxic, damaging learning, behavior, and health across the lifespan (Center on the Developing Child;
http://developingchild.harvard.edu/science/key-concepts/toxic-stress/).
Research on the epigenetic consequences of early-life adversity was spurred by experimental studies showing that variation in maternal care in early life was associated with epigenetic alterations in the brains of rodents (
12). Translation to humans has not been unchallenging. Although a substantial body of research has accumulated linking early-life adversity to differences in DNA methylation, methods and results are heterogeneous and nonoverlapping. Under the label of “adversity,” studies have focused on a diverse mix of exposures spanning maternal psychiatric disease (
13), early parental loss (
14), institutionalization (
15), indentured child labor (
16), child abuse (
17), and the Holocaust (
18). On the outcome side, DNA methylation differences in surrogate tissue, such as whole blood, have been reported, but often in different genomic regions. Moreover, because it is ethically impermissible to randomly assign human subjects to varying levels of adversity, observational studies have struggled to disentangle the effects of stress exposure from confounding effects of other toxins (e.g., tobacco smoking) and a host of other environmental (e.g., poverty) and genetic factors known to be correlated with stress exposure. More investigation of the link between stress exposure and DNA methylation is needed.
In this article, we report a test of the hypothesis that victimization is associated with DNA methylation in whole blood in a population-representative longitudinal study of a twin birth cohort followed to age 18. The study was explicitly designed—rather than conceived post hoc—to test for genomic consequences of victimization. To ensure the content validity of the measurement of threatening and violating stressful experiences, the study canvassed the multiple types of victimization that the young study participants encountered during development, both inside and outside the family, including physical and sexual abuse, emotional and physical neglect, and, as they grew older, bullying, cyber-victimization, and crime. Victimization was measured during adolescence (to test the impact of adverse events during the peak prevalence period of victimization), during childhood (to test the sensitive period of early-life adversity), and cumulatively across the first two decades of life (to test the cumulative stress load hypothesis of repeated and chronic stress). Using these exposure data, we report a two-pronged approach to discovery research on the epigenetics of stress. First, we conducted an epigenome-wide association study (EWAS) to test the hypothesis that victimization experiences are linked to methylation variation. Second, we interrogated variation in DNA methylation in the vicinity of candidate genes previously implicated in the stress response.
Method
Sample
Participants were members of the Environmental Risk (E-Risk) Longitudinal Twin Study, which tracks the development of a 1994–1995 birth cohort of 2,232 British children (
19). Briefly, the E-Risk sample was constructed in 1999 and 2000, when 1,116 families (93% of those eligible) with same-sex 5-year-old twins participated in home-visit assessments. This sample comprised 56% monozygotic and 44% dizygotic twin pairs, and sex was evenly distributed within zygosity (49% male). The study sample represents the full range of socioeconomic conditions in Great Britain, as reflected in the families’ distribution on a neighborhood-level socioeconomic index (ACORN [A Classification of Residential Neighborhoods], developed by CACI, Inc., for commercial use) (
20): 25.6% of E-Risk families live in “wealthy achiever” neighborhoods, compared with 25.3% nationwide; 5.3% compared with 11.6% in “urban prosperity” neighborhoods; 29.6% compared with 26.9% in “comfortably off” neighborhoods; 13.4% compared with 13.9% in “moderate means” neighborhoods; and 26.1% compared with 20.7% in “hard-pressed” neighborhoods. “Urban prosperity” neighborhoods are underrepresented in E-Risk because such households are often childless.
Home visits were conducted when participants were ages 5, 7, 10, 12, and, most recently, 18 years. Our epigenetic study used DNA from a single tissue: whole blood. At age 18, whole blood was collected in 10 mL K2EDTA tubes from 1,700 participants, and DNA was extracted from the buffy coat. (Study members who did not provide blood provided buccal swabs, but we did not include these in our methylation analysis, to avoid tissue-source confounders.) There were no differences in socioeconomic background, IQ, mental health, or victimization experiences between study members who participated and those who did not and between those who provided blood and those who did not. (For details, see the data supplement that accompanies the online edition of this article.)
Genome-Wide Quantification of DNA Methylation
Of 1,700 available blood samples, 31 were not usable (e.g., because of low DNA concentration), so we assayed 1,669 samples. Approximately 500 ng of DNA from each sample was treated with sodium bisulfite using the EZ-96 DNA Methylation Kit (Zymo Research, Irvine, Calif.). DNA methylation was quantified using the Illumina Infinium HumanMethylation450 BeadChip (Illumina 450K array) run on an Illumina iScan System (Illumina, San Diego, Calif.). Twin pairs were randomly assigned to bisulfite-conversion plates and Illumina 450K arrays, with siblings processed in adjacent positions to minimize batch effects.
Data were imported using the methylumIDAT function in the methylumi package (
21) and subjected to quality control analyses, checking for sex mismatches and for genotype data that did not concur with single-nucleotide polymorphisms (SNPs) typed on Illumina OmniExpress24v1.2 arrays, and excluding low-intensity samples (details are provided in the
online data supplement).
Samples from 1,658 participants passed our quality control pipeline, including 1,468 participants who were members of complete twin pairs (428 monozygotic pairs and 306 dizygotic pairs) and 190 participants whose co-twin did not have complete data (e.g., did not provide blood, did not pass quality control). Data were processed with the pfilter function from the wateRmelon package (
22), excluding zero samples with >1% of sites with a detection p value >0.05, 567 sites with bead count <3 in 5% of samples; and 1,448 probes with >1% of samples with a detection p value >0.05. The data were normalized with the dasen function from the wateRmelon package (
22). Before any analyses were conducted, probes with common SNPs (>5% minor allele frequency) within 10 bp of the single base extension and probes with sequences previously identified as potentially hybridizing to multiple genomic loci were excluded (
23,
24), resulting in a final data set of 430,802 probes.
Victimization Exposure
Childhood and adolescent victimization experiences in this cohort have been described previously (
2,
25) and are summarized here (details are provided in the
online data supplement).
Childhood victimization.
Childhood victimization was assessed repeatedly when the children were 5, 7, 10, and 12 years old, including exposure to intimate-partner violence between the mother and her partner, frequent bullying by peers, physical maltreatment by an adult, sexual abuse, emotional abuse and neglect, and physical neglect. Exposures were coded from 12-year dossiers for each child that comprised information from home-visit staff, mothers, children, family doctors, and child-protection interventions (see the
online data supplement). Each exposure across childhood was coded on a 3-point scale (0=no exposure, 1=probable/less severe exposure, 2=definite/severe exposure). Polyvictimization refers to the experience of multiple victimizations of different types, and it is a more powerful predictor of adverse outcomes than any particular exposure (
26). Following Finkelhor et al. (
26), we used the most straightforward and reproducible method of defining polyvictimization, operationalizing it as the simple count of different forms of victimization experienced by a child. All childhood victimization experiences coded as 2 were summed. Overall, 1,192 (71.9%) children had no severe victimization experiences, 355 (21.4%) had one, 70 (4.2%) had two, and 41 (2.5%) had three or more.
Adolescent victimization.
Adolescent victimization was assessed at age 18 when the twins were interviewed about experiences between ages 12 and 18 using the Juvenile Victimization Questionnaire (
27,
28), adapted as a clinical interview. Age 12 is a salient age for our participants because it is when British children leave primary school and enter secondary school. The Juvenile Victimization Questionnaire has good psychometric properties (
29), and it was used in the U.K. National Society for the Prevention of Cruelty to Children national survey (
30,
31), thereby providing benchmark values for comparisons with our cohort. The study assessed seven forms of victimization: maltreatment, neglect, sexual victimization, family violence, peer/sibling victimization, cyber-victimization, and crime victimization. Like childhood victimization, exposure to each type of adolescent victimization was coded on a 3-point scale (0=no exposure, 1=less severe exposure, 2=severe exposure) (see the
online data supplement). Adolescent polyvictimization was derived by summing all victimization experiences coded as 2. Overall, 1,064 (64.2%) adolescents had zero severe victimization experiences, 325 (19.6%) had one, 150 (9.2%) had two, and 118 (7.1%) had three or more.
Cumulative victimization.
We performed a latent class analysis combining the above-mentioned childhood and adolescent measures of victimization. Latent class analysis is a person-centered technique that classifies individuals into groups based on a profile of variables, in this case the degree of each participant’s exposure (none, moderate, or severe) to the six types of childhood and seven types of adolescent victimization. The latent class analysis was performed using only participants who experienced at least one form of victimization. It was conducted in MPlus, version 7.4, accounting for clustering of twins within families (see Table S1 in the online data supplement). The latent class analysis identified three victimized groups: 1) individuals who were exposed primarily to parental intimate-partner violence in childhood (N=254, 15%), 2) those who were primarily victimized by peers and street crime throughout childhood and adolescence (N=412, 24.8%), and 3) those who experienced multiple types of violence in both childhood and adolescence (N=158, 9.5%). For the analysis of cumulative victimization, we report a comparison of the most extreme groups from the 1,658 participants for whom methylation data were available: the 158 participants who were exposed to cumulative victimization across both childhood and adolescence and the 834 (50.3%) participants who were not exposed to childhood or adolescent victimization.
Twins’ retrospective self-reports of maltreatment during childhood.
In addition to the above prospectively ascertained measures of victimization, we assessed twins’ recall of victimization up to age 12 through the Childhood Trauma Questionnaire (
32), which participants completed at the age-18 follow-up. The questionnaire inquires about the severity of five forms of victimization: physical abuse, physical neglect, emotional neglect, sexual abuse, and emotional abuse. A total of 1,507 (91%) participants recalled zero moderate/severe victimization experiences, 97 (5.9%) recalled one, 25 (1.5%) recalled two, and 25 (1.5%) recalled three or more.
Statistical Analysis
Linear regression was used to test the association between victimization and DNA methylation variation. The model included the following covariates: sex, methylation-array control-probe principal components indexing technical variation, and cell-type proportion estimates (see the online data supplement). To control for known effects of smoking in methylation data, the model was refitted by adding information about smoking status as a covariate. Because the sample included members of twin pairs, we accounted for the nonindependence of observations by calculating robust standard errors using the R package gee. Complete results showing associations between victimization measures and each probe are available in a file deposited at Open Science Framework (osf.io/e9gdc).
We used regression to test the association between within-pair twin differences in victimization and within-pair differences in their DNA methylation, controlling for differences in blood cell–type proportion estimates and differences in smoking.
An array-wide significance threshold of p<1.16×10−7 was derived by applying a Bonferroni correction to the nominal alpha of 0.05, thereby adjusting for the 430,802 probes tested in the study.
We also interrogated candidate genes hypothesized to be involved in stress reactivity by identifying probes on the array that were annotated to prespecified genes. Probe sequences for the Infinium HumanMethylation450 BeadChip kit were aligned to the hg19 version of the human genome using the BLAT (
33) alignment algorithm. Probe sequences that mapped to multiple genomic loci were assigned to the genomic location provided by Illumina. Probe sequences that did not match any region of the genome with at least 94% identity were also assigned to the genomic location provided by Illumina. Each probe was then assigned to its nearest gene based on the GRCh37v75 ENSEMBL (
34) release of the human transcriptome. We report associations between victimization and these probes using both array-wide and gene-wide p value thresholds.
Regional Manhattan plots were generated using the R packages qqman and ggplot2 and the Bioconductor package ggbio.
Results
Victimization in the Peak Period of Adolescence
Adolescents are victimized by a more diverse set of actors across a wider range of environments than any other age group, and exposure to multiple types of victimization—including relational aggression, sexual victimization, and serious violent crime—peaks during adolescence (
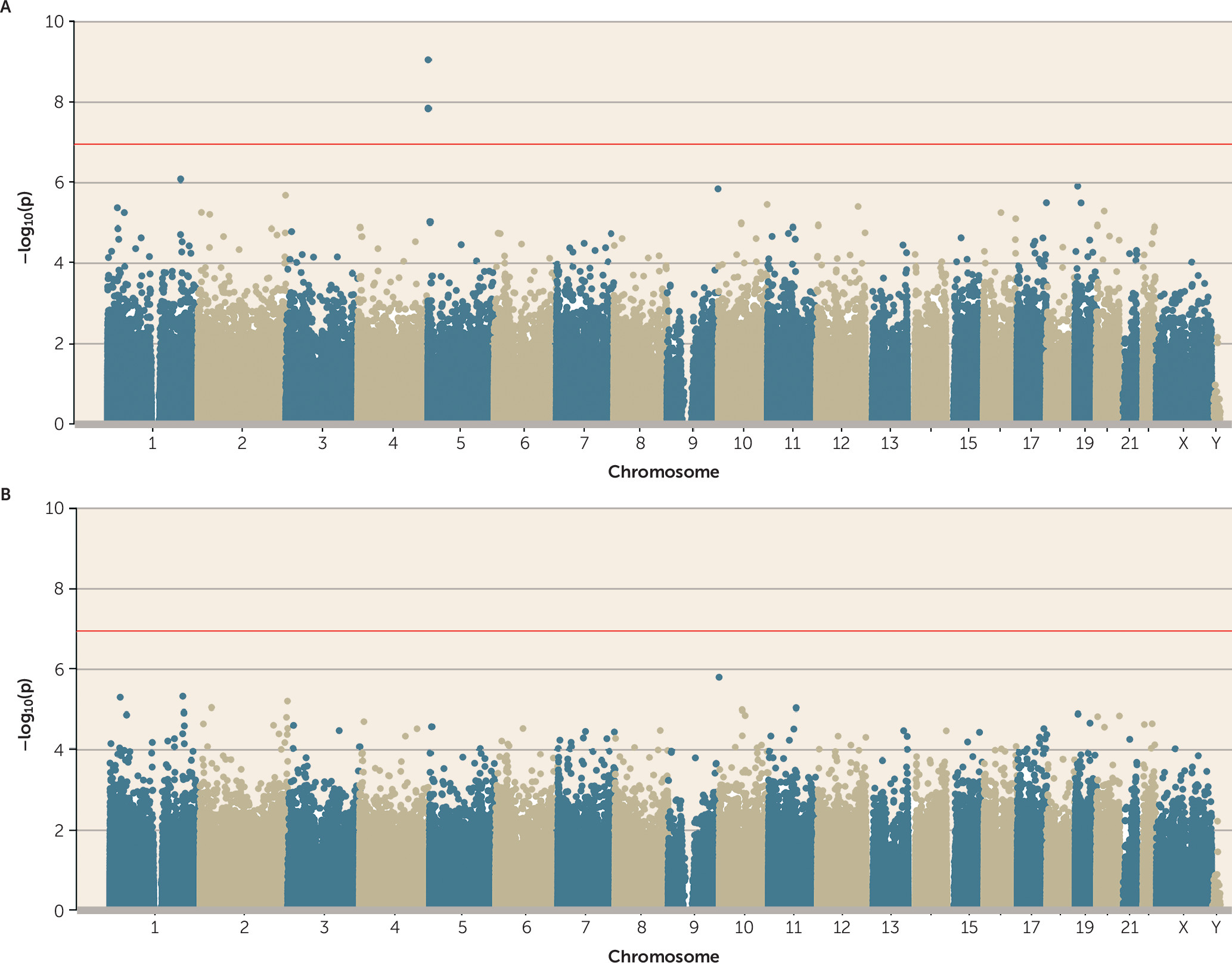
35). Therefore, we first tested whether polyvictimization during this peak period of exposure was associated with DNA methylation. Adolescent polyvictimization was significantly associated with DNA methylation (p<1.16×10
−7) at three differentially methylated positions (cg05575921, cg26703534, and cg21161138, all annotated to
AHRR) (
Figure 1A). Across all probes on the array, effect sizes were small (see the Open Science Framework file, osf.io/e9gdc), and these three probes were characterized by DNA methylation differences <1% for each additional type of victimization.
A prominent challenge to interpreting associations between victimization and DNA methylation is that victimized adolescents are more likely to smoke tobacco (
36), which has striking effects on DNA methylation (
37). We assessed smoking among E-Risk participants by calculating the number of pack-years that they smoked, observing that victimized adolescents were significantly more likely to smoke (p=2.51×10
−37) and to have smoked more pack-years (p=4.92×10
−14) (
Figure 2A). In the E-Risk sample we also replicate the finding that tobacco smoking is associated with multiple genome-wide changes in DNA methylation, with 83 probes meeting the array-wide significance threshold (
Figure 2B; see Table S2 in the
online data supplement for more details). When smoking pack-years was added as a covariate to the polyvictimization EWAS, no probes remained significantly associated with polyvictimization at an array-wide significance threshold (
Figure 1B). This is not surprising, because the three array-wide significant probes from the polyvictimization EWAS—those annotated to
AHRR—were also among the probes significantly associated with tobacco smoking in a large recent EWAS (
37) as well as in the EWAS of pack-years in E-Risk (
Figure 2C).
Of course, not all types of victimization are alike. Some involve physical injury, whereas others involve psychological insult; some are immediate, others remote (e.g., cyberbullying); some are perpetrated by strangers, others by people known to the victim. Our measure of polyvictimization may have diluted the effects of specific forms of victimization. Thus, we next tested the association between exposure to each victimization type (i.e., maltreatment, neglect, sexual victimization, family violence, peer/sibling victimization, cyber-victimization, and crime victimization) and DNA methylation (see Figure S1 in the online data supplement). The results revealed few novel associations and sparsely distributed significant findings across the seven types of victimization. We detected a total of eight array-wide significant associations, but none of the eight were shared between victimization types. Two of the eight (cg05575921 and cg21161138, both annotated to AHRR and confounded by smoking) were among the three previously identified in our analysis of adolescent polyvictimization.
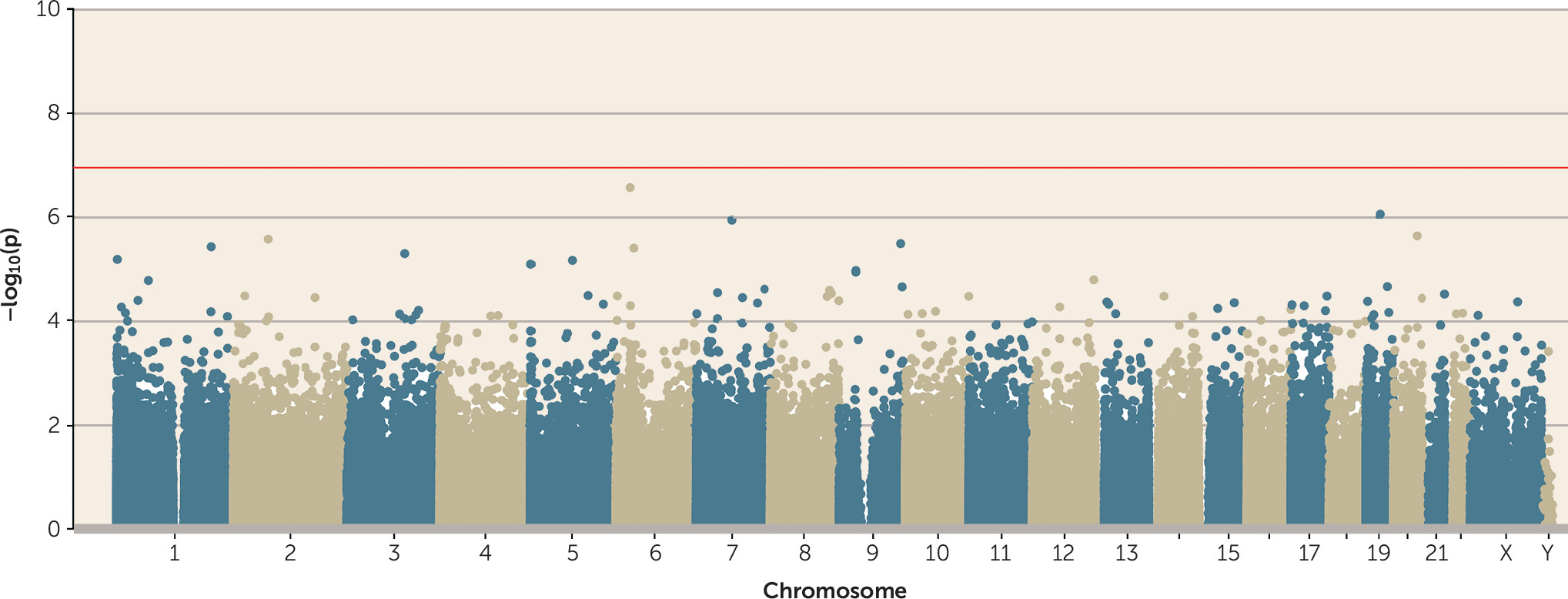
Testing the Sensitive Period of Childhood Victimization
Although most victimization experiences peak in adolescence, it has been hypothesized that the most biologically consequential victimization is experienced earlier in life (
38). However, no probes passed the array-wide significance threshold in a regression model of childhood polyvictimization (
Figure 3).
As with adolescent polyvictimization, we tested associations between exposure to each of the six victimization types (physical abuse, physical neglect, emotional abuse/neglect, sexual abuse, intimate-partner violence, and bullying victimization) and DNA methylation (see Figure S2 in the online data supplement). A total of 48 array-wide significant associations were observed across four of the six victimization types (physical abuse, emotional abuse/neglect, sexual abuse, and intimate-partner violence). None of these probes were shared between victimization types, and none of them were identified in the EWAS of childhood polyvictimization. Interestingly, of these 48 probes, 39 were associated with childhood sexual victimization. These probes are listed in Table S3 in the data supplement. These findings indicate that childhood sexual victimization is associated with stable DNA methylation differences in whole blood in young adulthood. However, these findings should be interpreted with caution because few children had recorded sexual victimization (N=29), and these associations were not observed in relation to sexual victimization in adolescence (see Table S3 in the data supplement).
Testing the Cumulative Stress Load Hypothesis
Perhaps the most consequential stressors are those that are experienced chronically or recurrently, and revictimization is a striking finding in epidemiology (
39). For example, in E-Risk, every type of victimization in childhood was associated with a significantly greater risk of victimization in adolescence (
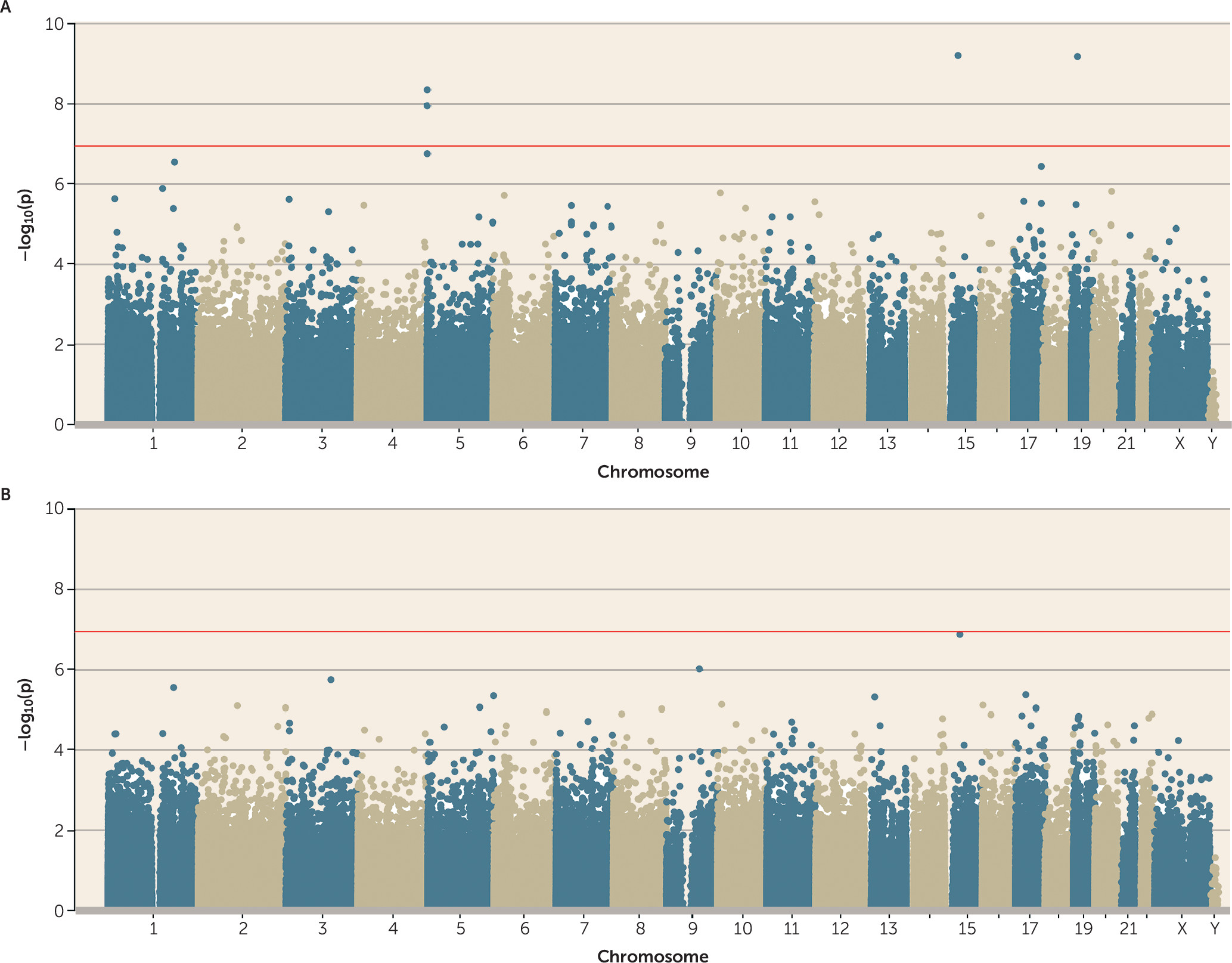
25), and polyvictimized children were 1.60 (95% CI=1.42, 1.82) times more likely to be polyvictimized again as adolescents. This suggested the hypothesis that the biological embedding of victimization is especially likely to occur in response to a greater cumulative stress load. The latent class representing cumulative polyvictimization was significantly associated with four CpG probes (cg05575921 and cg21161138, both annotated to
AHRR; cg00944304, annotated to
BAHD1; and cg03636183, annotated to
F2RL3) (
Figure 4A). Two of these were the same
AHRR probes that were previously found to be associated with adolescent polyvictimization (see
Figure 1A). None of these probes remained array-wide significant after controlling for smoking (
Figure 4B).
Are Retrospective Reports of Childhood Victimization in Young Adulthood Associated With Epigenetic Variation?
Some evidence linking childhood adversity with DNA methylation comes from cross-sectional studies of adults who retrospectively report on their childhood experiences (
40). Moreover, some research suggests that the association between childhood maltreatment and mental health problems is stronger when maltreatment is retrospectively recalled than when it is ascertained prospectively (
41,
42). We thus extended our analysis to test whether associations would emerge when childhood victimization was measured at age 18 with the Childhood Trauma Questionnaire (
32), a popular tool for retrospectively assessing childhood maltreatment history in adults. Similar to what has been observed in other studies (
43,
44), in E-Risk there was only fair agreement between prospective and retrospective reports of childhood maltreatment (weighted kappa=0.21), raising the possibility that different operationalizations of exposure may yield different findings.
Retrospective reports of childhood victimization were significantly associated with two probes, neither of which had been identified in any of the previous analyses (cg03960390, annotated to
RER1; and cg07146173, annotated to
ALKBH5) (
Figure 5A). After controlling for smoking, both remained array-wide significant (
Figure 5B). We carried these two probes forward to a co-twin control analysis that tested whether the more victimized twin was differentially methylated relative to his or her less victimized co-twin. Neither of these probes remained statistically significant at an alpha of 0.025 (correction for two tests) in a co-twin control model (p values, 0.06 and 0.09, respectively), which suggests that the association between victimization and methylation is possibly confounded by shared genetic and/or environmental factors.
As with the other measures of polyvictimization, we tested associations between each of the five Childhood Trauma Questionnaire victimization types (physical abuse, physical neglect, emotional neglect, sexual abuse, and emotional abuse) and DNA methylation (see Figure S3 in the online data supplement). A total of 48 array-wide significant associations were observed across all five victimization types. None of these probes were shared between victimization types. Of these 48 probes, 22 were associated with retrospective reports of childhood sexual abuse. However, the probes associated with Childhood Trauma Questionnaire reports of childhood sexual abuse did not overlap with probes associated with prospectively ascertained reports of childhood sexual victimization. Table S3 in the data supplement lists all the probes associated with sexual victimization (as measured during childhood, during adolescence, and retrospectively in young adulthood) and shows that DNA methylation patterns associated with sexual victimization were not reproducible across different operationalizations of this stressor.
To further investigate associations between retrospective Childhood Trauma Questionnaire reports of childhood victimization and DNA methylation, we turned to comparable data from the Dunedin Longitudinal Study (
45), which followed a 1972–1973 birth cohort to age 38. At the age-38 assessment, we administered the same Childhood Trauma Questionnaire measure as in E-Risk, assayed DNA methylation from blood samples using the same Illumina 450K array, and conducted parallel analyses of 818 Dunedin study members with complete data (details are provided in the
data supplement). Two findings stand out. First, after controlling for smoking, no probes passed the array-wide significance threshold in a regression of DNA methylation on retrospective Childhood Trauma Questionnaire reports of childhood victimization (see Figure S4 in the
data supplement). Second, none of the 22 probes that were associated with retrospective reports of childhood sexual abuse in the E-Risk sample met the significance threshold in the Dunedin sample (based on a Bonferroni correction for number of probes tested [0.05/22=0.002]) (see Table S3 in the
data supplement).
Epigenetic Interrogation of Known Stress-Related Genes
An epigenome-wide analysis overlooks cumulative evidence about the biological plausibility of specific candidate genes. In particular, genes involved in hypothalamic-pituitary-adrenal (HPA) axis reactivity may be differentially methylated in response to victimization. These loci include
NR3C1 (the glucocorticoid receptor, which binds cortisol and triggers its downstream effects on gene expression) (
46),
FKBP5 (a regulator of the glucocorticoid receptor network) (
47),
BDNF (the gene encoding brain-derived neurotrophic factor, a member of the nerve growth factor family) (
48),
AVP (the gene encoding the neuropeptide vasopressin, which is secreted as part of the HPA response to stress) (
49), and
CRHR1 (the corticotropin-releasing hormone receptor, another major player in the HPA pathway) (
50). In addition, genetic variants annotated to
SLC6A4 (the serotonin transporter gene) have been implicated in stress reactivity, and these effects may be mediated by altered DNA methylation (
51).
We first identified all probes on the Illumina 450K array annotated to each of these six genes. The number of probes per gene ranged from 16 to 66. We then examined associations between these probes and adolescent, childhood, and cumulative victimization, as well as retrospective Childhood Trauma Questionnaire reports of childhood victimization.
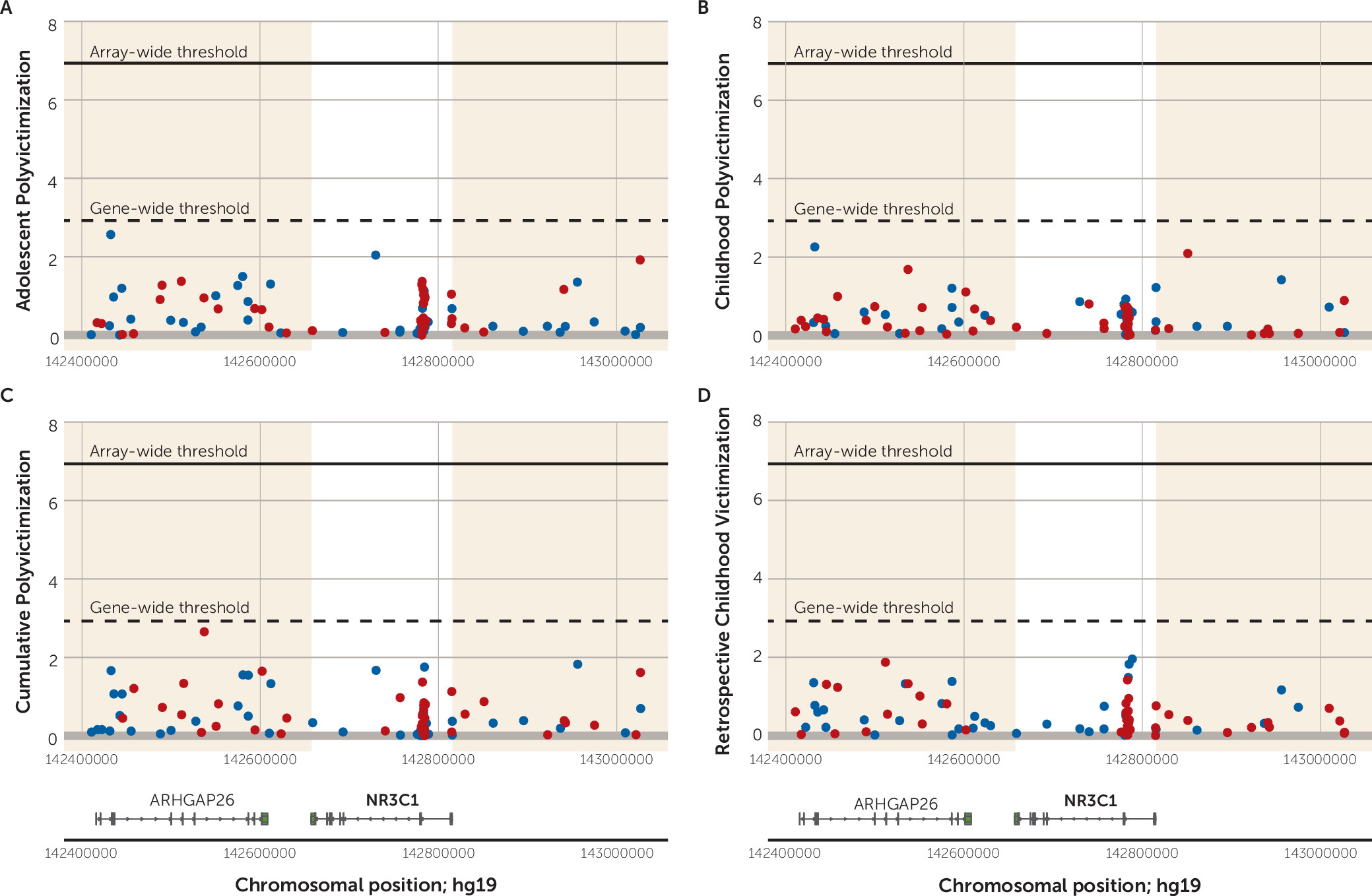
To illustrate,
Figure 6 shows the regional Manhattan plot of −log
10 p values and effect directions (negative [hypo-] versus positive [hyper-] methylation) for all probes annotated to
NR3C1. (The plot shows probes annotated to the genomic region surrounding
NR3C1, and the gene-wide significance threshold is based on a Bonferroni correction for the number of probes annotated to the gene.) For
NR3C1, we observed no gene-wide significant probes associated with victimization within the
NR3C1 gene region. The regional Manhattan plots for the remaining five candidate genes are presented in Figures S5–S9 in the
online data supplement. Overall, looking across the six candidate genes and the four victimization exposures, only four probes crossed the threshold for gene-wide significance (
AVP cg23035419 [p=0.0016] and cg25551168 [p=0.0019] in relation to adolescent polyvictimization;
BDNF cg20954537 [p=0.0002] in relation to Childhood Trauma Questionnaire reports of polyvictimization; and
FKBP5 cg00140191 [p=0.0004] in relation to childhood polyvictimization). Individual association statistics for all probes annotated to the six genes, in relation to each of the four victimization exposures, are listed in the Open Science Framework file (osf.io/e9gdc), and Figures S5–S9 in the
data supplement show significance values for probes in the regions surrounding each gene as well.
Discussion
This study offers, to our knowledge, the most comprehensive analysis to date of epigenetic alterations in humans’ response to victimization stress in the first two decades of life. The study design contains five strengths intended to enhance internal and external validity. First, it is the largest sample yet to test the association of victimization with epigenetic variation in whole blood. Second, it minimized both ascertainment and attrition bias; the study followed a nationwide birth cohort that represents the full range of socioeconomic conditions in Great Britain with no referral bias or selective attrition. Third, the study collected valid detailed measures of multiple forms of victimization in childhood and in adolescence and ascertained cumulative victimization across the first two decades of life. Fourth, it introduced controls for smoking, which may generate spurious epigenetic associations with psychosocial factors. Fifth, it was able to use a family-based design to compare twin siblings growing up in the same households to test confounding effects of shared environmental and genetic risk factors.
Results from both epigenome-wide association analyses and from interrogation of candidate genes involved in the human stress response revealed limited evidence for an association between victimization and epigenetic variation in peripheral blood. Three contributions to the literature stand out. First, relative to nonpsychosocial toxins, such as tobacco (see Table S2 in the online data supplement), methylation associations with “toxic” psychological stress are less pervasive and small. Second, methylation associations identified for victimization overlapped with those identified for tobacco smoking, highlighting difficulties in disentangling biological effects of psychosocial influences from effects of health behaviors associated with a victimization history. Third, the few methylation associations that were identified for victimization did not replicate across different specifications of victimization stress, including ascertainment periods and reporting sources, or in an additional replication sample.
Guided by the hypothesis that the epigenome mediates the effects of stress on poor health (
52), epigenetic variation detected in blood is hypothesized to represent an informative window into the study of biological embedding (
48). For this to be true, it would be important to observe epigenetic differences between victimized children and their nonvictimized peers that were either moderate in size or pervasive across the genome. Results from this study are not consistent with the presence of such epigenetic differences in peripheral blood.
One of the most important questions regarding the present study is how the findings should be interpreted in the context of existing research on biological embedding of early-life stress and childhood maltreatment. This is particularly salient given reports that do document epigenetic influences of stressful experiences.
First, it is possible that the lack of associations observed in this study constitute a false negative. However, we had invested heavily in this project, were surprised by the dearth of findings, and were motivated to turn our data upside down in an effort to detect significant associations. In addition to “epigenome-wide” analyses, we carried out candidate-gene association analyses; we tested associations with victimization in different age periods as well as cumulative victimization across multiple age periods; we disaggregated our index of polyvictimization in order to test associations with multiple different specific forms of victimization, including physical abuse, sexual abuse, and crime victimization; we used victimization data from self and others; we presented findings at different significance thresholds, ranging from array-wide p values to gene-wide p values. Against the background of these efforts, the results did not yield consistent reproducible findings.
Second, it is possible that our exposure measurement was inadequate, because of either unreliability or poor content validity. However, we used reliable, valid, and age-appropriate methods to assesses victimization, and exposed cases in our cohort were known to have endured severe experiences. Moreover, the victimization experiences we analyzed predict poor psychiatric outcomes strongly in this cohort (
3), attesting that it is possible to detect stress-related consequences of victimization using this study’s victimization measures. Specifically, victimization was followed by increases in mental health problems over a childhood previctimization baseline of emotional/behavioral problems, and discordant-twin analyses showed that victimization predicted an increased risk of mental health problems independently of family background and genetic risk. On the outcome side, our methylation data replicate the same clear signatures of cigarette smoking that have been observed in other studies (
37) (see Table S2 in the
online data supplement), attesting that it is possible to detect epigenetic modifications related to environmental exposures using this study’s methylation data.
Third, it is possible that previous reports contained some false positive findings or more circumscribed findings. Many epigenetic studies of stress are very small, running the risk that effect sizes for statistically significant associations could be overestimated (
53). Other studies do not always correct significance thresholds for multiple testing when evaluating epigenetic associations, or they filter the probes in ways resulting in too lenient correction. And, in some instances, positive epigenetic associations of early-life stress sometimes depend on genotype, on trauma exposure, or on the presence of psychiatric disorder (
47,
54–
56). In general, the replication record of previous findings is difficult to evaluate and summarize because different studies have examined different organisms, different age groups, different stressors, and different genomic regions, sometimes in different tissues (brain, blood, saliva, or buccal cells). Additionally, variation in technological approaches introduces a lack of parallelism in the type of methylation data produced (e.g., high-throughput array-based technologies versus lower-throughput pyrosequencing). Although there is an overall impression of links between childhood adversity and epigenetic variation, the consistency may be more apparent than real.
The lack of association in this large epidemiological study between victimization and epigenetic variation in blood raises several considerations for evaluating future studies on the epigenetics of stress.
First, seminal epigenetic research on biological embedding of stress used brain tissue in rodents (
12), focusing on genomic regions of interest. However, with notable exceptions (
46,
57), the vast majority of subsequent human studies, including the present one, have relied on peripheral blood. This choice is expedient, but also scientifically reasonable given the aim of detecting effects on stress-related physical health systems that include peripheral circulating processes (immune, neuroendocrine). But whole blood is heterogeneous, and although cell-type composition can be evaluated and controlled, as in the present study, it does raise the question of whether peripheral blood is a problematic surrogate tissue for research on the epigenetics of stress (
58). Comparisons of methylomic variation across blood and brain suggest that blood-based EWAS may yield limited information relating to underlying pathological processes for disorders where brain is the primary tissue of interest (
59).
Second, this study tested the “main effect” hypothesis that victimization experiences in childhood lead to epigenetic modifications. It is quite possible that the answer to this question is more nuanced. For example, exposure-related methylation signatures may be concentrated only among the subset of victimized individuals who develop stress-related disorders (
60) or could be enhanced by risk factors or mitigated by protective factors (
47). If the epigenetic effects of stress are so nuanced, the challenge will be in drawing conclusions from studies of a range of disorders and of multiple risk and protective factors.
Third, researchers who seek to survey DNA methylation across the epigenome are limited to using commercial arrays whose probes interrogate only a proportion of all CpG sites in the epigenome. In this regard, it is important to note that key areas of the epigenome reported to index maltreatment exposure are sparsely covered on the Illumina array (
40,
61). For example, no probes from the Illumina platform map to intron 7 of
FKBP5 (
47), and the few probes that map to the
NR3C1 noncoding promoter region (
46) were not significant in our study. Thus, the absence, in this study, of evidence for methylation differences in candidate genes that have been associated with victimization does not translate to evidence of the absence of methylation differences in these or other genes. Moreover, while epigenetic modifications may play a mediating role in translating and establishing the long-term health outcomes associated with early-life stress and victimization, DNA methylation may not be the only epigenetic modification directly involved in this process (
62,
63). More generally, it is important not to lose sight of the well-documented association between early-life victimization and poor adult health (
8,
9) and of the need to uncover how this link comes about, not only through various biological factors but also through psychological and lifestyle factors.
Fourth, observational studies are perennially challenged in getting a handle on the temporal nature of biological effects of stress. Unlike in experimental studies in model organisms—where stress can be manipulated (e.g., through glucocorticoid administration or maternal separation) and its epigenetic effects monitored at preset intervals—in epigenetic epidemiological studies of human stress, including the present one, stress exposure is often correlated with genetic differences, methylation variation is typically assessed at one point in time, and the gap between exposure and biological measurement is highly variable and often indeterminate (
64). Environmentally induced DNA modifications do not necessarily persist throughout the life course (
65), and it is not known when and for whom epigenetic modification becomes biologically stabilized after stress exposure. This creates both design and interpretive challenges in documenting and describing dynamic responses to early childhood adversities.
In summary, the findings from this comprehensive epidemiological analysis of the epigenetic effects of early-life stress do not support the hypothesis of robust changes in DNA methylation in victimized young people. Our conclusion is not that DNA methylation is unimportant. Rather, it is that observational studies using free-ranging humans, relying on peripheral tissue, and using currently available high-throughput technologies appear to yield weak and inconsistent evidence on the epigenetics of early-life stress. We need to come to terms with the possibility that epigenetic epidemiology is not yet well matched to nonhuman experimental models in uncovering how stress gets under the skin in humans.