Borderline personality disorder is characterized by hyperreactivity in response to emotional stimuli, severe affective instability, impaired interpersonal relationships, and emotion dysregulation (
1–
4). The emotional dysregulation has great significance clinically, because it affects patients’ social functioning, life satisfaction, and identity (
1,
5). Although research has begun to elucidate the neurobiological correlates underlying emotional hypersensitivity among patients with borderline personality disorder in single sessions (
6,
7), an important unanswered question investigated here concerns whether and how these responses are affected as the same negative stimuli are reencountered. This question is of particular ecological relevance because emotionally salient situations typically present themselves not once but recurrently over a span of days or longer.
One adaptive, typical response to repeated negative stimulus presentation, over the short or long term, is habituation (
8,
9), which relies on well-established mechanisms at the cellular level involving depression of synaptic transmission (
10,
11). Cellular response habituation represents an important physiological mechanism implicated in psychological processes such as extinction of conditioned responses (
12). Although few studies of extinction learning using classical conditioning paradigms exist for borderline personality disorder, one recent study pointed to delayed extinction among individuals with borderline personality disorder (
13). Additionally, and importantly, habituation has been implicated as a key mechanism of action for many desensitization-based psychotherapies, including prolonged exposure therapy (
14,
15).
Indeed, for some people, and under some circumstances, responses do not decrease with repeated presentation but rather increase (i.e., sensitize). One useful theoretical framework views psychological and physiological responses to repeated stimulation as a dual process: a plasticity of response that involves separable component processes of habituation and sensitization (
8). Although the tendency toward habituation responses is dependent on factors that include the number of identical repetitions, the tendency toward sensitization responses is increased for repeated stimuli that are particularly aversive (i.e., particularly salient) to the recipient of the stimulation (
16). Thus, we examined the longitudinal profile of psychological and neurophysiological responses to repeated presentation of negative stimuli that were particularly germane to the phenomenology of borderline personality disorder. Additionally, we examined these processes among healthy adult control subjects and patients with avoidant personality disorder, a group which, given that it constitutes a near-neighbor personality disorder involving hypersensitivity in response to negative evaluation (
17), as well as intermediate negative emotional reactivity relative to patients with borderline personality disorder and healthy control subjects (
18), represents a psychopathological control group. Although comparative behavioral evidence is minimal, both patients with borderline and avoidant personality disorders have not been shown to exhibit habituation of negative affect self-reports in response to a single repetition of a negative image in an individual session, in contrast to healthy control subjects (
18).
Given that the intense shifts in affect that characterize borderline personality disorder pertain to external or internal cues perceived as meaningful, we chose to focus on activation in the neural salience network (
19), comprising the amygdala, anterior insula, and dorsal anterior cingulate cortex. The salience network is involved in integrating sensory, emotional, and cognitive information to filter out and amplify information through both bottom-up and top-down means (
19–
21). The amygdala is a key player in this regard, with a prominent role in assessing the salience (and in the case of negative information, the threat value) of a stimulus (
22,
23). The anterior insula has been implicated in highly diverse psychological functions related to salience, including sensory integration and awareness (
20,
24). Furthermore, the dorsal anterior cingulate cortex has been associated with cognitive control, performance monitoring, and executive attention (
22,
25).
For patients with borderline personality disorder and healthy control subjects, a recent meta-analysis of neuroimaging studies of reactivity to emotional stimuli showed that of the aforementioned salience network regions, the most consistent findings have been observed in the amygdala, with most studies indicating amygdala hyperactivation during appraisal of emotional stimuli (i.e., faces and scenes) (
6). Another recent neuroimaging meta-analysis of differences in neural activation among patients with borderline personality disorder compared with healthy control subjects during emotion processing similarly suggested consistent evidence of amygdala hyperactivation among patients with borderline personality disorder (
26). Meta-analysis results of group differences in emotion reactivity in the anterior insula and anterior cingulate cortex among patients with borderline personality disorder and healthy control subjects have been more mixed (
6,
26). For the anterior insula, a quantitative meta-analysis (
26) indicated hyperactivation among patients with borderline personality disorder compared with healthy control subjects, whereas two of nine studies analyzed in another meta-analysis (
6) reported hypoactivation in this region among patients with borderline personality disorder compared with healthy control subjects, with the other seven studies not indicating a result.
Although borderline personality disorder has been examined in a relatively small but growing number of functional neuroimaging studies, only two such studies, to our knowledge, have examined avoidant personality disorder in any context (
18,
27). Results demonstrated evidence of amygdala hyperactivation during anticipation of engagement in emotion regulation among patients with avoidant personality disorder compared with healthy control subjects (
27) as well as underrecruitment of the dorsal anterior cingulate cortex in the context of habituation in response to a single repetition of a negative stimulus (
18). Patients with borderline personality disorder were likewise shown to exhibit dorsal anterior cingulate cortex hypoactivation compared with healthy control subjects in the aforementioned single-repetition habituation paradigm (
18).
Therefore, given the clinical relevance of examining habituation processes over a period longer than a single repetition of a negative stimulus, our principal focus here was to test whether and how patients with borderline personality disorder differ from patients with avoidant personality disorder and healthy control subjects in terms of longitudinal habituation and sensitization in response to several presentations of a negative stimulus over multiple days, as measured by both functional MRI (fMRI) and trial-by-trial self-reports of negative affect. In terms of neural activity, our principal focus was on habituation or sensitization of the salience network as a whole, with the amygdala, anterior insula, and dorsal anterior cingulate cortex regions defined anatomically and independently of the study data. We predicted that negative affect reports and salience network activity would demonstrate hyperactivation among patients with borderline personality disorder compared with patients with avoidant personality disorder and healthy control subjects, manifesting either as diminished longitudinal response habituation or anomalous longitudinal response sensitization to negative stimuli.
Method
Participants
We recruited patients with borderline personality disorder (N=30), patients with avoidant personality disorder (N=31), and healthy control subjects (N=33) from outpatient clinics at the Mount Sinai Medical Center and the James J. Peters VA Medical Center in New York City as well as from newspaper and online advertisements. All participants provided written informed consent after procedures were fully explained. Exclusions due to motion, signal quality, and related issues are summarized in the online supplement. A total of 19 participants were excluded (borderline personality disorder, N=4; avoidant personality disorder, N=6; and healthy control, N=9), yielding a total of 75 participants for analyses (borderline personality disorder, N=26, mean age=37.0 years [SD=10.0], 15 females; avoidant personality disorder, N=25, mean age=37.2 years [SD=9.8], 13 females; healthy control, N=24, mean age=32.6 years [SD=7.9], 11 females). There were no significant differences in age by group (F=1.92, df=2, 72, p=0.15, pairwise t tests were not significant), and there was no significant difference in gender ratio (χ2=0.70, df=2, 75, p=0.70). The three groups did not differ in years of education (borderline personality disorder group: 14.6 years [SD=2.4]; avoidant personality disorder group: 14.3 years [SD=2.8]; healthy control group: 15.6 years [SD=3.3], pairwise two-tailed t tests were not significant).
Participants with borderline personality disorder met DSM-IV criteria for borderline personality disorder, including the affective instability criterion. Participants with avoidant personality disorder met DSM-IV criteria for avoidant personality disorder but not for borderline personality disorder. Patients with borderline and avoidant personality disorders did not meet DSM-IV criteria for past or present bipolar I disorder, schizophrenia, or schizoaffective disorder. Patients with avoidant personality disorder did not meet criteria for past or present posttraumatic stress disorder. Comorbidities present in the two patient groups are summarized in the
online supplement. Participants in all groups were free of any psychotropic medication for at least 2 weeks (6 weeks for fluoxetine) before the start of the study. Healthy control subjects did not meet DSM-IV criteria for any axis I or II disorder. Diagnostic assessments were obtained with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (
28), and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (
29). Our research group has achieved an interrater reliability of 0.81 for diagnosing borderline personality disorder.
Materials
Thirty negative images and 30 neutral images were used. All images (negative and neutral) were social, depicting two or more people interacting. All negative images contained themes specifically relevant to borderline personality disorder, including interpersonal rejection, sadness, frustration, anger, and violence. Images were drawn by using the Empathy Picture System (
30) and online image repositories, and they were rated with the Self-Assessment Manikin (
31) for comparability (see the
online supplement).
Task Design
Participants completed a habituation task during fMRI scanning at two separate sessions. At session 1, participants viewed five presentations of 30 negative and 30 neutral task images, divided into three equally sized functional runs of 10 negative images (five presentations of each) and 10 neutral images (five presentations of each). Thus, a total of 100 image presentations occurred per each of the three runs (50 image presentations per valence per run), and a total of 300 image presentations occurred overall at session 1. The image presentation order was pseudorandomly counterbalanced across participants. Each trial consisted of an image presentation (3 seconds), a negative affect rating (3 seconds), and a brief fixation interval (1 second). During image presentation, participants were instructed to look at each image and respond naturally, keeping their eyes on the image the entire time. During the negative affect rating period, participants were instructed to provide a rating of their current level of negative affect on a scale of 1–5 (1=least negative; 5=most negative).
Session 2 followed session 1 by approximately 3 days. At session 2, participants returned to the fMRI scanner and viewed the same images, presented in the original order, that they had seen at session 1.
Data Acquisition and Analysis
Self-reported negative affect.
Self-reported negative affect ratings were acquired by using a five-button response glove during fMRI scanning and recorded with E-Prime software (Psychology Software Tools, Sharpsburg, Pa.). Self-reported affect data were analyzed by using linear mixed models incorporating fixed-effect estimates for group (borderline personality disorder, avoidant personality disorder, and healthy control), session (1 or 2), valence (negative or neutral), and presentation order (1, 2, 3, 4, and 5) and their interactions as well as a random effect consisting of an intercept for each participant.
fMRI.
Whole-brain fMRI data were acquired by using a 3.0-T Philips Achieva scanner (Philips Healthcare, Amsterdam) (for acquisition and preprocessing information, see the online supplement). A random-effects general linear model was computed with regressors corresponding to the interaction of three conditions (session, valence, and presentation order [for further details, see the online supplement]).
Anatomical definitions of salience network regions of interest.
Because our primary hypotheses concerned activation of a priori–defined regions in the salience network, we defined regions of interest for the right and left amygdalae, right and left anterior insulae, and dorsal anterior cingulate cortex by using standard anatomical atlases (
32,
33). Details of each region of interest are provided in the
online supplement.
In order to provide a unified test of our hypotheses, we constructed a network activity estimate, because our hypotheses principally concerned salience network activity as a whole. Although component anatomically defined regions of interest varied in size (see the online supplement), all five regions of interest were hypothesized to contribute equally to salience network activity. Therefore, salience network activity was defined as the average of activity estimates for each of the five component regions of interest (i.e., the right and left amygdalae, right and left anterior insulae, and dorsal anterior cingulate cortex). Neural activity was further examined within each region of interest in particular. Neural activity was then analyzed with linear mixed models by using the aforementioned factors described for analysis of self-reported negative affect ratings.
Results
Self-Reported Negative Affect
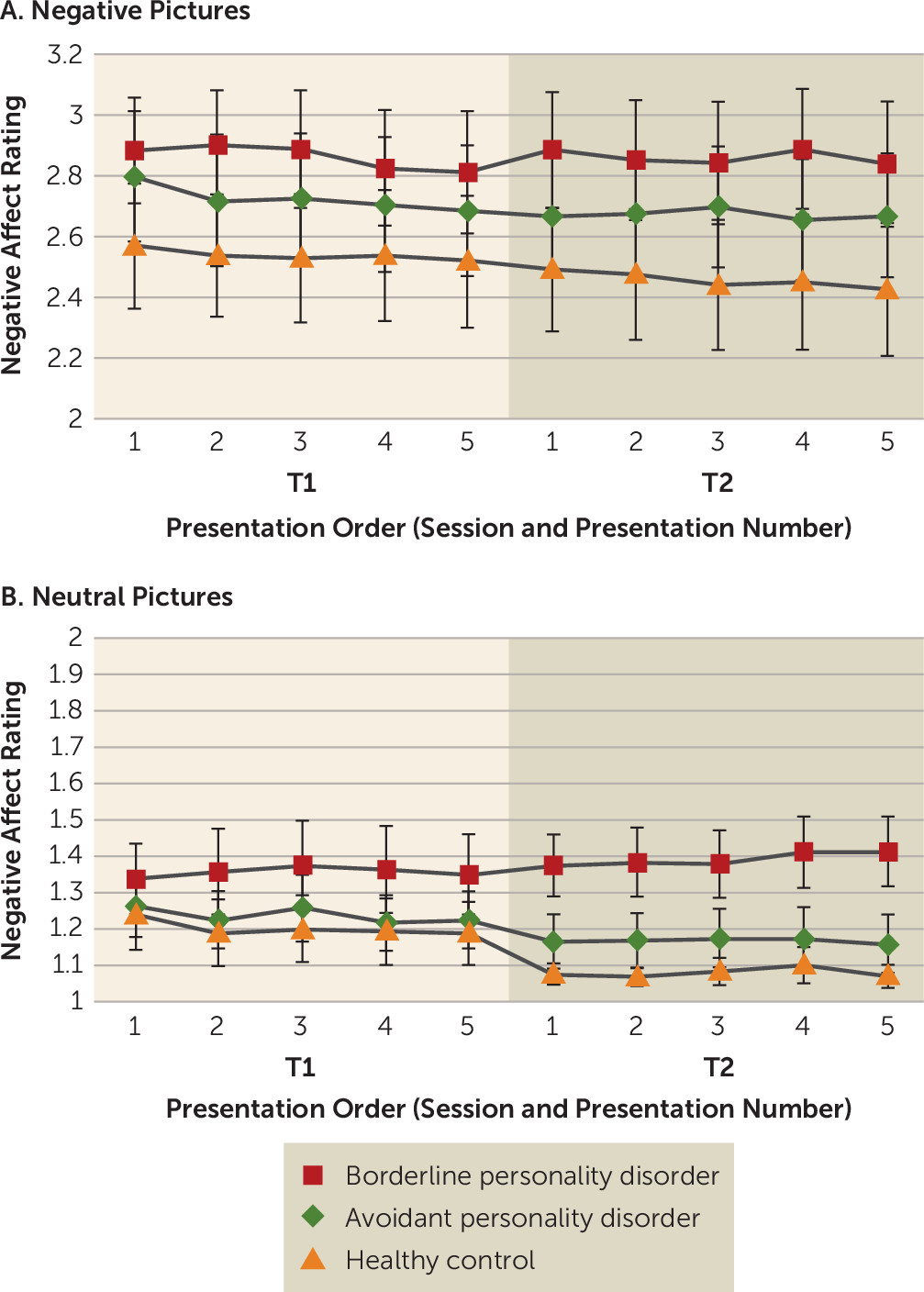
Negative-affect self-reports during the habituation task are summarized in
Figure 1. As expected, a main effect of valence was present (negative stimuli > neutral stimuli) (F=2814.31, df=1, 1368, p<0.01). There was no significant effect of presentation order (F=0.15, df=4, 1368, n.s.), indicating that there was little within-session habituation overall. However, a marginal main effect of session that fell short of statistical significance was present (session 2 < session 1) (F=3.18, df=1, 1368, p<0.08), indicating global habituation across sessions. Furthermore, a marginal group-by-valence interaction that also fell short of significance was present (F=2.70, df=2, 1368, p<0.07).
In order to analyze this interaction, and given our hypotheses concerning responses to negative images in particular, we examined responses in each valence individually. For negative images (
Figure 1A), there was a main effect of session, indicating habituation across sessions overall (F=8.73, df=1, 648, p<0.01). Additionally, a marginal group-by-session interaction was present, indicating a tendency of patients with borderline personality disorder to show less change over time (i.e., diminished habituation) compared with patients with avoidant personality disorder and healthy control subjects (F=2.50, df=2, 648, p<0.09). This is demonstrated by within-group comparisons showing that change over time was not significant in the borderline personality disorder group (neither from the first to the last presentation [t=0.51, df=25, p=0.62, two-tailed, n.s.; Bayes factor=0.15, Bayes factor <1, indicating that the null finding was not driven by data insensitivity] nor from the average of the first session to the second session [t=0.00, df=25, p=0.99, two-tailed, n.s.; Bayes factor=0.11]), whereas a decrease in negativity over time from the first to last presentation was significant in the healthy control group (t=2.27, df=23, p<0.04, two-tailed) (
34). Patients with avoidant personality disorder showed session-1 habituation that fell short of significance (t=1.88, df=24, p<0.08, two-tailed) but no between-session habituation (t=0.71, df=24, p=0.48, two-tailed, n.s.), nor did they show a difference from the first to last presentation (t=1.38, df=24, p=0.18, two-tailed).
Similarly, for neutral images (
Figure 1B), there was a main effect of session (F=16.46, df=1, 648, p<0.01), indicating global between-session habituation. Furthermore, there was a significant group-by-session interaction (F=13.16, df=2, 648, p<0.01). Here, healthy control subjects showed a marginal decrease in negative affect from the first to last presentation (t=1.98, df=23, p<0.07, two-tailed), and patients with avoidant personality disorder also showed a marginal decrease (t=2.06, df=24, p<0.06, two-tailed), whereas patients with borderline personality disorder showed an anomalous tendency toward sensitization over time (t=1.45, df=25, p<0.09, one-tailed).
fMRI
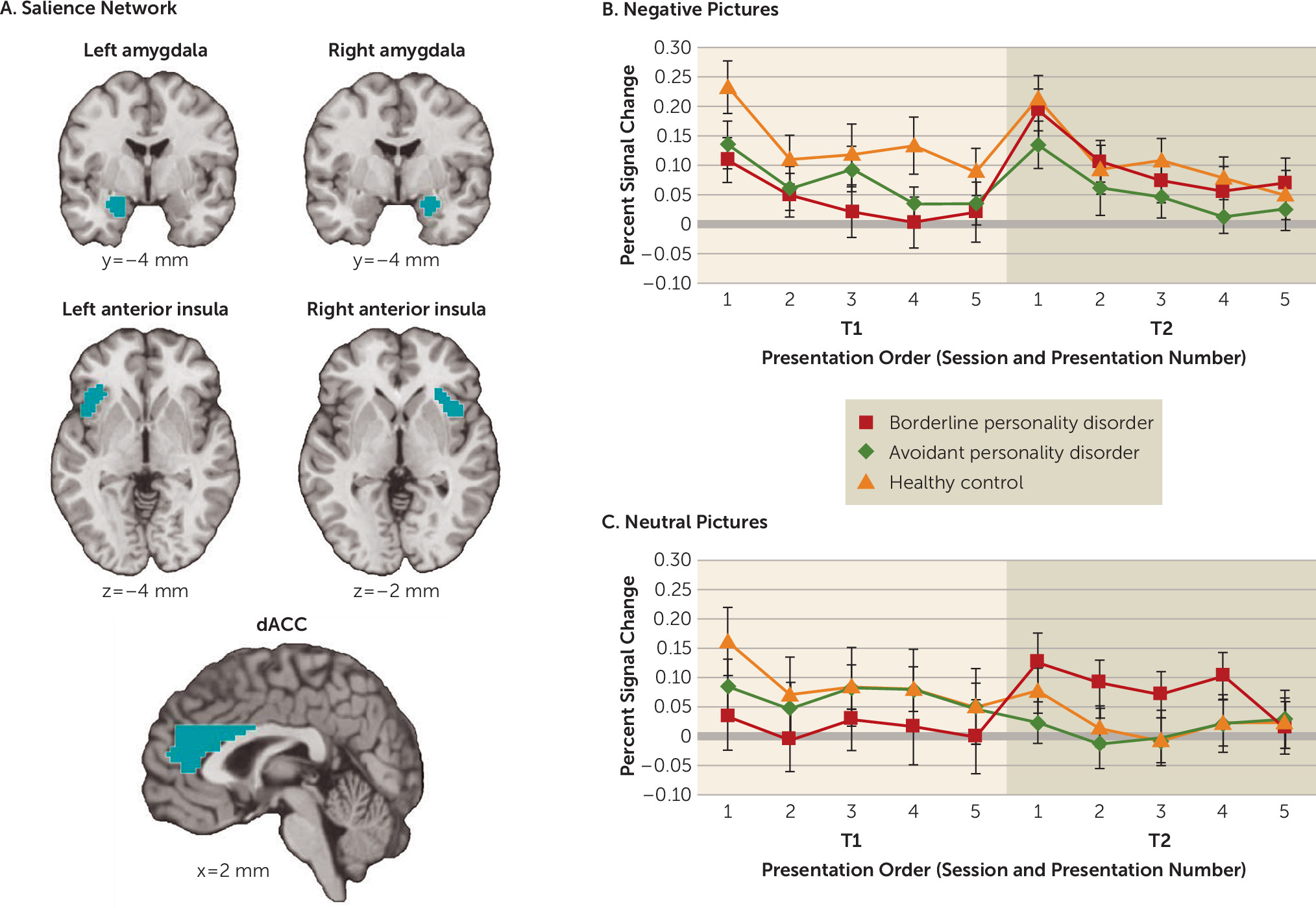
Salience network activity.
In order to examine habituation and sensitization processes in the brain, we examined the overall activity in the salience network (
Figure 2). In addition to a main effect of valence (negative stimuli > neutral stimuli) (F=16.37, df=1, 1368, p<0.01), there was a main effect of presentation order (F=11.55, df=4, 1368, p<0.01), reflecting significant within-session habituation overall across all groups (see the
online supplement). Furthermore, there was a significant valence-by-presentation order interaction (F=2.50, df=4, 1368, p<0.05), indicating greater within-session habituation in response to negative images compared with neutral images overall.
Importantly, there was a significant group-by-session interaction (F=14.74, df=2, 1368, p<0.01), with anomalous between-session sensitization overall observed in the borderline personality disorder group. Given our particular hypotheses regarding responses to negative images, we investigated this interaction for each valence separately. Indeed, this anomalous between-session sensitization of salience network activity was present for negative images (F=6.90, df=2, 648, p<0.01) and neutral images (F=8.16, df=2, 648, p<0.01). For negative images, this interaction was demonstrated by anomalous elevated salience network activity across sessions (i.e., between-session sensitization), which fell short of significance, among patients with borderline personality disorder (t=1.69, df=25, p<0.06, one-tailed), whereas there was no change in activity across sessions among healthy control subjects (t=0.81, df=23, n.s.) or patients with avoidant personality disorder (t=0.44, df=24, n.s.).
As an exploratory analysis, we further examined self-reported negative affect and salience network activity among patients with avoidant personality disorder as a function of the presence or absence of comorbidity with social phobia (i.e., the comorbidity with the largest sample size across participants, as 11 patients with avoidant personality disorder had social phobia; for further details, see the online supplement, including Figure S1).
Salience network component regions of interest.
Although our primary interest was in assessing activity in the salience network overall, we further investigated activity in each of the anatomically defined salience network component regions of interest individually (for right amygdala, left amygdala, right anterior insula, left anterior insula, and dorsal anterior cingulate cortex activity, respectively, see the online supplement, including Figures S2–S6). As described in the online supplement, these results further characterize the aforementioned salience network results, with broadly consistent results across regions, but with evidence of a laterality asymmetry. Specifically, activity in the right amygdala, right anterior insula, and dorsal anterior cingulate cortex demonstrated the aforementioned group-by-session interaction, with anomalous between-session sensitization of response in the borderline personality disorder group, whereas activity in the left amygdala and left anterior insula did not show significant change across sessions among patients with borderline personality disorder (see the online supplement).
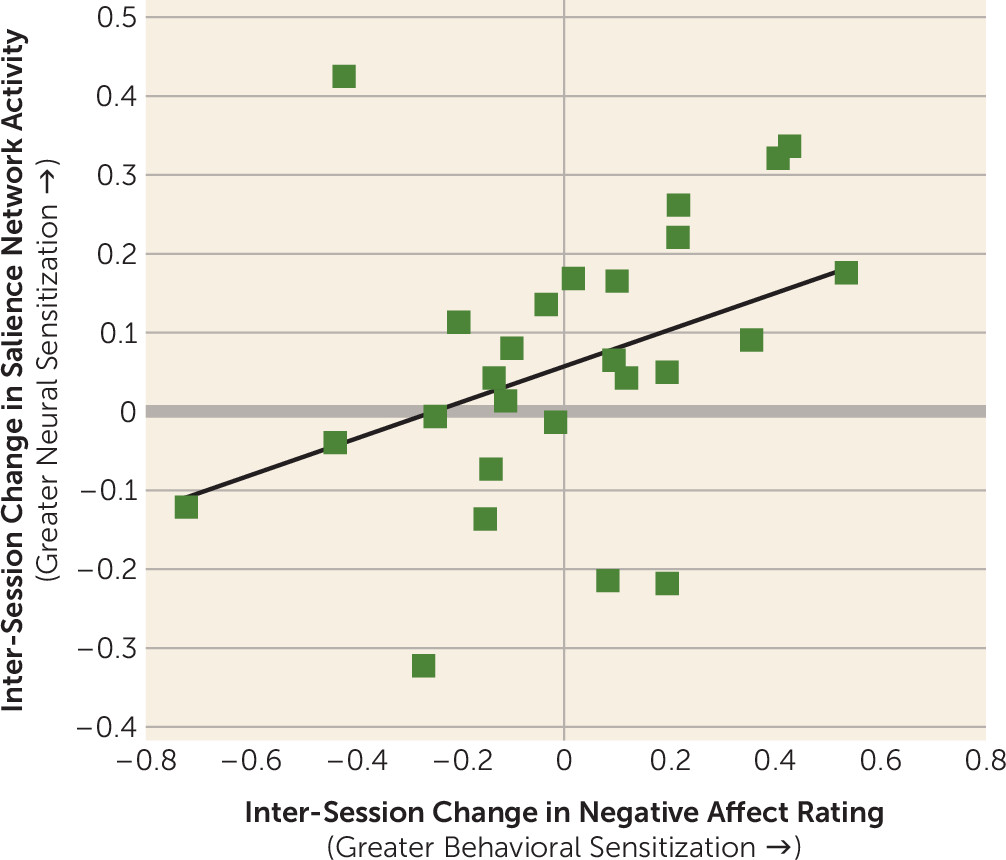
Correlation Between Self-Reported Negative Affect and Salience Network Activity Among Patients With Borderline Personality Disorder
A brain-behavior correlation was observed in the borderline personality disorder group, with greater longitudinal sensitization of self-reported negative affect ratings (i.e., greater average response in session 2 compared with session 1 in response to negative images) predicting greater longitudinal sensitization of salience network activity defined in the same way (r=0.38, p<0.03, one-tailed) (
Figure 3).
In an exploratory whole-brain analysis, no additional regions met multiple-comparison correction criteria for significant sensitization or habituation across sessions (1 and 2) for either valence (negative and neutral) in any group (see the online supplement).
Discussion
In this study, we examined anomalies in within- and between-session habituation and sensitization—dual processes that are clinically relevant to the phenomenology of borderline personality disorder—in patients with borderline and avoidant personality disorders and in healthy control subjects. Given previous research examining emotional reactivity in response to negative stimuli among patients with borderline personality disorder and healthy control subjects, we hypothesized that self-reports of negative affect and activity in the salience network (i.e., including the amygdala, anterior insula, and dorsal anterior cingulate cortex [
19,
21]) would be differentially recruited across groups with regard to habituation and sensitization profiles, in particular by reflecting either diminished habituation or exaggerated sensitization in the borderline personality disorder group.
Although our results support comparable within-session habituation responses across groups, we found evidence of diminished habituation and anomalous sensitization in the borderline personality disorder group between sessions. The self-reported negative affect data supported this hypothesis in that patients with borderline personality disorder showed diminished habituation of response across sessions for both negative and neutral social images (i.e., diminished habituation in response to negative images and, for neutral images, an anomalous marginal tendency toward an increase in self-reported negative affect over the course of the experiment). Additionally, salience network activity supported our hypothesis regarding anomalous longitudinal sensitization in the borderline personality disorder group. Even though all groups exhibited initial within-session habituation, only patients with borderline personality disorder showed a between-session increase in salience network activity. As further validation of the present fMRI results, we found evidence that between sessions, behavioral sensitization (i.e., increased negative affect reports) predicted salience network sensitization among patients with borderline personality disorder. These results are consistent with the role of the salience network in assigning value and importance to environmental stimuli (
19,
21–
23).
Interestingly, this between-session salience network sensitization pattern was observed for neutral images, potentially suggesting top-down negative emotion generation in the borderline personality disorder group, whereby even neutral social stimuli are appraised as relatively negative (
35,
36). Thus, the longitudinal sensitization observed among patients with borderline personality disorder in response to neutral and negative social stimuli may reflect anomalous negative appraisal processes in borderline personality disorder, inferring negativity in social situations when it does not objectively exist. This interpretation is consistent with the observed self-reported negative affect data for neutral images, which showed diminished habituation and anomalously negative overall negative affect reports in response to neutral images among patients with borderline personality disorder compared with patients with avoidant personality disorder and healthy control subjects.
Although our primary focus was on activity in the salience network as a whole, it is noteworthy that laterality played a role in the results regarding the salience network. Between-session sensitization effects in the borderline personality disorder group were observed in only one laterality for the amygdala and anterior insula (i.e., in the right hemisphere only). At the same time, an exploratory laterality analysis indicated that in this experiment, left-hemisphere responses to the social picture stimuli in these regions were stronger overall than right-hemisphere responses across groups and valences. In spite of the fact that some evidence among healthy adults has suggested that the right hemisphere may be relatively attuned to negative (compared with positive) stimulus processing (
37–
39), some quantitative meta-analyses have called the reliability of this effect into question (
40), and consistent with the laterality results presented here, a true account may be more nuanced (
41) and depend on person factors (e.g., group) as well as context.
The need for the use of appropriate clinical control groups has been highlighted in the literature to establish psychopathological specificity (
6). Here, we examined patients with avoidant personality disorder in parallel with patients with borderline personality disorder and healthy control subjects. Previous work has shown that patients with avoidant personality disorder exhibit intermediate negative reactivity compared with patients with borderline personality disorder and healthy control subjects (
18), which is supported in the negative affect results here. Overall, however, patients with avoidant personality disorder showed longitudinal neural response profiles in the salience network that resembled the profiles observed in the healthy control group but not the borderline personality disorder group. Although clinically near-neighbors, avoidant and borderline personality disorders may draw on somewhat divergent neural networks (
18). Furthermore, from a dimensional perspective, patients with avoidant personality disorder may not have reached the threshold for maladaptive reactivity that is associated with anomalous sensitization in the manner shown by patients with borderline personality disorder.
Additionally, even though this was not a conditioning study, the salience network results in the borderline personality disorder group shown here may be relevant to the phenomenon of spontaneous recovery in extinction paradigms (
12,
13,
42). Although the patients with borderline personality disorder showed initial habituation responses that paralleled those of patients with avoidant personality disorder and healthy control subjects, the anomalous sensitization exhibited by the borderline personality disorder group may be reflective of the reemergence of a salience response to particularly population-relevant stimuli following a delay of approximately 3 days.
Conclusions
This study suggests that individuals with borderline personality disorder may ascribe hypersalient significance to repeated negative social stimuli through hyperactivation of the neural salience network. Awareness of evidence for longitudinal sensitization in borderline personality disorder may have implications for the psychotherapy used as a treatment modality for this disorder. For example, it may help patients with borderline personality disorder and psychotherapists to better understand and prepare for the not uncommon experience of reactions intensifying rather than subsiding when salient emotional episodes are revisited in successive psychotherapy sessions. This could have implications for the optimal timing of therapeutic interventions or militate against treatment approaches predicated heavily on systematic desensitization strategies.
Acknowledgments
The authors thank Dr. Larry J. Siever.