Doubly Randomized Preference Trial of Prolonged Exposure Versus Sertraline for Treatment of PTSD
Abstract
Objective:
Method:
Results:
Conclusions:
Method
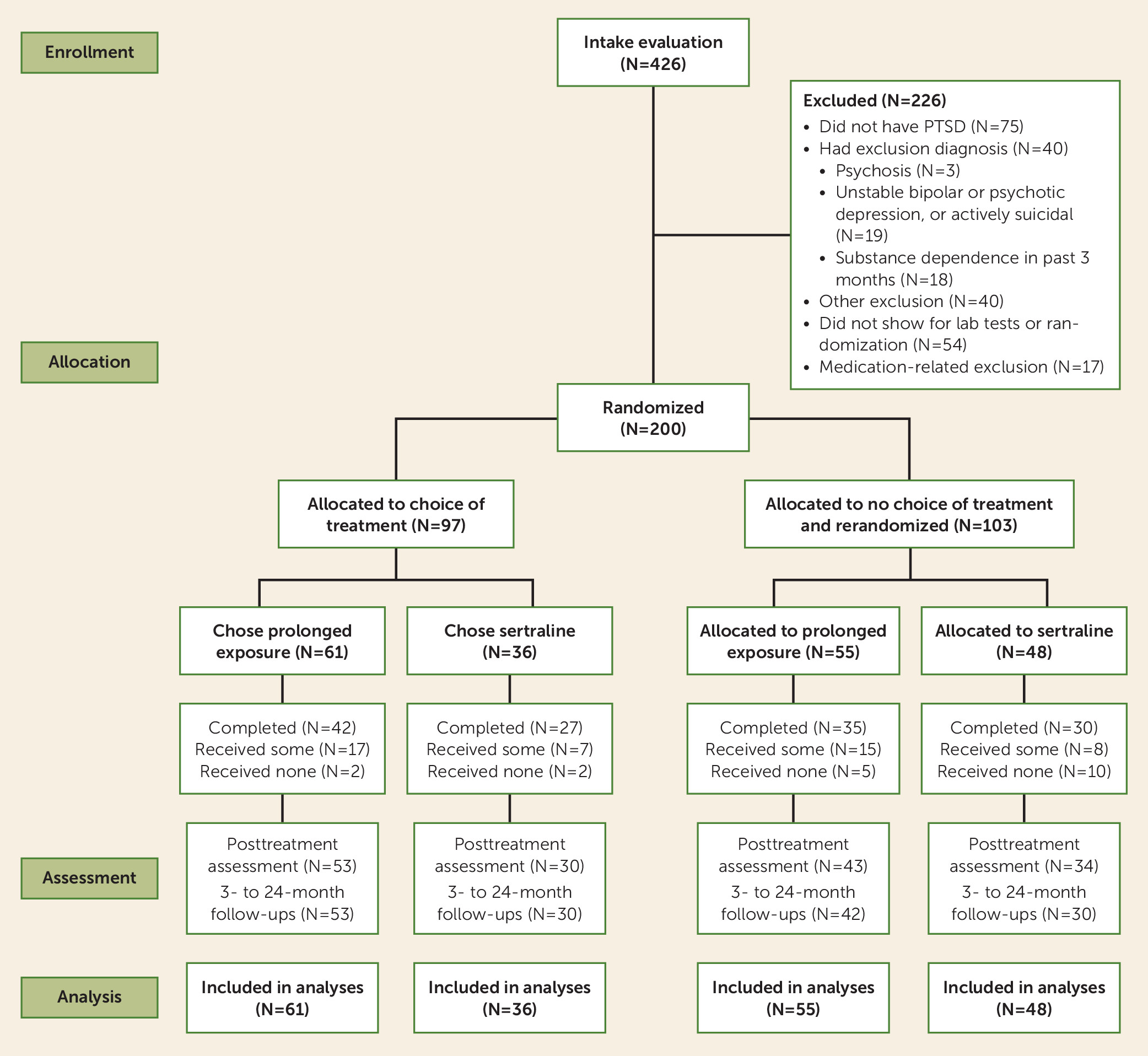
Study Design and Participants
Measures
Procedures
| Characteristic | ||
|---|---|---|
| Mean | SD | |
| Age (years) | 37.41 | 11.30 |
| Time since trauma exposure (years) | 11.97 | 12.69 |
| N | % | |
| Gender | ||
| Female | 151 | 75.5 |
| Male | 49 | 24.5 |
| Ethnicity | ||
| Caucasian | 131 | 65.5 |
| African American | 43 | 21.5 |
| Other | 26 | 13.0 |
| Not college educated | 140 | 70.0 |
| Income <$20,000/year | 97 | 48.5 |
| Trauma type | ||
| Adult sexual assault | 62 | 31.0 |
| Childhood assault (e.g., childhood sexual abuse) | 48 | 24.0 |
| Adult assault (nonsexual) | 45 | 22.5 |
| Accident (e.g., motor vehicle crash, natural disaster) | 27 | 13.5 |
| Death or violence to loved one | 13 | 6.5 |
| Combat or war | 5 | 2.5 |
| Mean | SD | |
| Number of other DSM-IV criterion A events | 9.05 | 6.23 |
| N | % | |
| Axis I co-occurrence | ||
| Current | 134 | 67.0 |
| Lifetime | 192 | 96.0 |
| Previous psychiatric treatment | ||
| Any treatment | 170 | 85.0 |
| Inpatient treatment | 38 | 19.0 |
Pretreatment assessment.
Videotaped treatment rationales.
Randomization and masking.
Pretreatment, posttreatment, and follow-up assessments.
Interventions
Sertraline.
Prolonged exposure.
Supervision and treatment integrity.
Adverse events.
Data Analysis
Results
Prolonged Exposure Compared With Sertraline
Acute outcome: pretreatment to posttreatment assessments.
| Treatment Condition and Measure | Pretreatment Assessment | Posttreatment Assessment | 24-Month Follow-Up Assessment | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Prolonged exposure | ||||||
| PTSD Symptom Scale–Interview | 29.4 | 29.1–29.7 | 10.5 | 9.5–11.5 | 8.0 | 6.6–9.3 |
| Reexperiencing | 7.6 | 7.5–7.6 | 2.5 | 2.3–2.6 | 1.4 | 1.3–1.6 |
| Avoidance | 12.3 | 12.2–12.3 | 4.1 | 3.7–4.5 | 3.3 | 2.7–4.0 |
| Arousal | 9.6 | 9.5–9.7 | 4.0 | 3.8–4.3 | 3.0 | 2.6–3.5 |
| PTSD Symptom Scale–Self Report | 33.9 | 32.6–35.2 | 13.8 | 11.8–15.7 | 12.1 | 10.5–13.8 |
| N | % | N | % | N | % | |
| PTSD diagnosis | 116 | 100.0 | 36 | 31.0 | 36 | 31.0 |
| Responder status | 0 | 0.0 | 84 | 72.4 | 85 | 73.3 |
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Hamilton Depression Rating Scale (24-item) | 22.8 | 22.4–23.2 | 11.5 | 11.1–12.0 | 9.7 | 8.5–10.9 |
| Beck Depression Inventory | 24.2 | 22.5–25.9 | 9.3 | 7.8–10.9 | 9.2 | 7.8–10.6 |
| Spielberger State Anxiety Inventory–State | 54.8 | 53.0–56.5 | 38.4 | 36.4–40.4 | 39.1 | 37.1–41.1 |
| Sheehan Disability Scale | 18.0 | 16.8–19.1 | 10.1 | 8.9–11.4 | 8.2 | 6.9–9.4 |
| Sertraline | ||||||
| PTSD Symptom Scale–Interview | 29.8 | 29.5–30.1 | 13.3 | 11.9–14.7 | 11.2 | 9.4–13.0 |
| Reexperiencing | 7.5 | 7.5–7.6 | 2.9 | 2.7–3.1 | 1.6 | 1.4–1.8 |
| Avoidance | 12.1 | 12.0–12.2 | 5.3 | 4.8–5.8 | 4.5 | 3.7–5.3 |
| Arousal | 10.2 | 10.1–10.3 | 5.1 | 4.7–5.5 | 4.9 | 4.2–5.7 |
| PTSD Symptom Scale–Self Report | 35.8 | 34.4–37.3 | 17.0 | 14.4–19.5 | 15.9 | 13.9–17.9 |
| N | % | N | % | N | % | |
| PTSD diagnosis | 84 | 100.0 | 38 | 45.2 | 38 | 45.2 |
| Responder status | 0 | 0.0 | 46 | 54.8 | 50 | 59.5 |
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Hamilton Depression Rating Scale (24-item) | 25.0 | 24.5–25.4 | 13.7 | 13.1–14.3 | 12.5 | 10.8–14.2 |
| Beck Depression Inventory | 25.8 | 23.9–27.8 | 13.1 | 10.7–15.6 | 13.1 | 10.9–15.3 |
| Spielberger State Anxiety Inventory–State | 57.2 | 55.3–59.1 | 43.5 | 40.6–46.3 | 42.5 | 40.0–45.0 |
| Sheehan Disability Scale | 19.3 | 18.0–20.7 | 12.5 | 10.7–14.4 | 10.2 | 8.5–11.9 |
Maintenance of gains: posttreatment to 24-month follow-up assessment.
Patient Treatment Preference
Acute outcome: pretreatment to posttreatment assessment.
| Measure | Pretreatment Assessment | Posttreatment Assessment | 24-Month Follow-Up Assessment | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Match with preference | ||||||
| PTSD Symptom Scale–Interview | 29.5 | 29.3–29.7 | 10.8 | 9.9–11.7 | 9.4 | 8.1–10.6 |
| Reexperiencing | 7.5 | 7.5–7.6 | 2.4 | 2.3–2.5 | 1.6 | 1.4–1.7 |
| Avoidance | 12.3 | 12.2–12.4 | 4.4 | 4.0–4.7 | 3.9 | 3.3–4.5 |
| Arousal | 9.6 | 9.5–9.7 | 4.1 | 3.9–4.3 | 3.8 | 3.3–4.3 |
| PTSD Symptom Scale–Self Report | 34.5 | 33.3–35.7 | 13.9 | 12.2–15.6 | 13.3 | 11.9–14.8 |
| N | % | N | % | N | % | |
| PTSD diagnosis | 149 | 100.0 | 44 | 29.5 | 48 | 32.2 |
| Responder status | 0 | 0.0 | 108 | 72.5 | 109 | 73.2 |
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Hamilton Depression Rating Scale (24-item) | 23.7 | 23.4–24.0 | 11.8 | 11.4–12.2 | 11.2 | 10.1–12.4 |
| Beck Depression Inventory | 24.8 | 23.4–26.2 | 10.0 | 8.6–11.4 | 10.4 | 9.0–11.7 |
| Spielberger State Anxiety Inventory–State | 55.9 | 54.4–57.4 | 39.6 | 37.8–41.4 | 40.7 | 38.9–42.5 |
| Sheehan Disability Scale | 18.7 | 17.7–19.7 | 11.3 | 10.0–12.6 | 9.1 | 7.9–10.3 |
| Mismatch with preference | ||||||
| PTSD Symptom Scale–Interview | 29.7 | 29.3–30.1 | 14.7 | 12.8–16.6 | 8.4 | 6.3–10.6 |
| Reexperiencing | 7.5 | 7.4–7.6 | 3.6 | 3.4–3.8 | 1.2 | 0.9–1.4 |
| Avoidance | 12.1 | 11.9–12.3 | 5.4 | 4.7–6.1 | 3.4 | 2.4–4.3 |
| Arousal | 10.4 | 10.3–10.5 | 5.7 | 5.2–6.2 | 3.7 | 2.8–4.6 |
| PTSD Symptom Scale–Self Report | 35.4 | 33.7–37.1 | 19.6 | 16.2–23.0 | 14.0 | 11.3–16.7 |
| N | % | N | % | N | % | |
| PTSD diagnosis | 51 | 100.0 | 30 | 58.8 | 26 | 51.0 |
| Responder status | 0 | 0.0 | 22 | 43.1 | 26 | 51.0 |
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Hamilton Depression Rating Scale (24-item) | 23.9 | 23.3–24.5 | 14.4 | 13.6–15.2 | 9.5 | 7.6–11.4 |
| Beck Depression Inventory | 25.3 | 22.5–28.1 | 14.3 | 10.9–17.7 | 12.3 | 9.4–15.3 |
| Spielberger State Anxiety Inventory–State | 55.4 | 52.6–58.2 | 44.3 | 40.5–48.2 | 38.8 | 35.7–42.0 |
| Sheehan Disability Scale | 18.1 | 16.4–19.8 | 10.4 | 8.4–12.4 | 8.6 | 6.8–10.4 |
Maintenance of gains: posttreatment to 24-month follow-up assessment.
Treatment Adherence
| Measure | Match With Preference | Mismatch With Preference | p | d or NNT | Mean Difference or RR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Completed treatment | 109 | 73.2 | 25 | 49.0 | 0.002 | 4.1 | 0.53 | 0.36–0.77 |
| Dropped out after randomization | 6 | 4.0 | 13 | 25.5 | <0.001 | 4.7 | 0.16 | 0.06–0.39 |
| Dropped out after <4 sessions | 23 | 15.4 | 23 | 45.1 | <0.001 | 3.4 | 0.34 | 0.21–0.55 |
| Mean | SD | Mean | SD | |||||
| Sessions completed | 7.69 | 3.19 | 5.14 | 4.26 | <0.001 | 0.67 | 2.55 | 1.44–3.67 |
| Sertraline dosage (mg/day) | 144.20 | 66.46 | 62.03 | 69.63 | <0.001 | 1.21 | 82.17 | 51.55–112.79 |
| Adherence scoresb | ||||||||
| Sertraline | 21.98 | 12.84 | 12.67 | 13.58 | 0.002 | 0.70 | 9.32 | 3.38–15.25 |
| Prolonged exposure in vivo exposure homework | 19.53 | 11.71 | 13.57 | 14.56 | 0.046 | 0.45 | 5.95 | 0.98–11.81 |
| Prolonged exposure imaginal exposure homework | 15.21 | 9.71 | 10.19 | 10.73 | 0.037 | 0.49 | 5.02 | 0.30–9.75 |
Post Hoc Analysis: Treatment Modality and Patient Preference
Discussion
Acknowledgments
Footnotes
Supplementary Material
- View/Download
- 147.70 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

