Previous meta-analyses that included data on reward processing and depression have differed from this work in various aspects, including a focus on selected age groups (for example, excluding patients under 18) or on limited populations or only on patients with severe depression; analysis of region-of-interest-based studies; and use of lenient thresholds; some of these studies are also now outdated (
6–
10). Similarly, no previous quantitative review has pooled effects of electrophysiological studies exploring the association between reward processing and depression. While EEG’s spatial resolution is inferior to that of functional MRI (fMRI), its superior temporal resolution is particularly relevant to the study of reward processing dynamics. Moreover, feedback-related negativity (FRN; also termed reward positivity) has emerged as a powerful measure of reward processing (
11,
12) implicated in depression (
13), making it essential to include such studies. Notably, in this meta-analysis, we also focused on developmental effects, as both reward processes (
14) and depression show developmental moderation (
15).
Conceptual Links Between Depression and Reward Processing
Cardinal presentations of depression (
16), most notably anhedonia, are thought to reflect alterations of the experience of reward (
17,
18). The following paragraphs conceptually bridge clinical terminology with the burgeoning science of reward processing.
Rewards have been defined as stimuli that induce behaviors that help the animal organism obtain what is necessary for survival (
19). In addition, rewards and punishers facilitate learning through positive or negative reinforcement: a reward (or lack of punishers) following a behavior will make the future occurrence of that behavior more likely, often eliciting feelings of pleasure; the opposite is true for behaviors followed by punishers (or lack of rewards). Reductions in reports of pleasure and approach-related behavior are a prominent feature of depression, and many suggest that they arise from aberrations in reward processing (
3,
20).
In
Table 1, we have adapted previous models (
21,
22) to parse four sets of reward processing events and map their links to clinical phenomena. We term the first stage of reward processing
prediction: it encompasses recognizing an object as potentially rewarding, a process that involves using existing knowledge about the value of objects. Anticipatory anhedonia, defined as a lack of interest in activities that used to be enjoyable, is the clinical depressive symptom that best maps onto this phase. In translational terms, this phase is typically captured by the reward or loss anticipation/prediction phase of an experiment, when a stimulus induces the subject to expect either a win or a loss. When attempting to engage prediction-related processes in translational work, the classic task is the monetary incentive delay paradigm (
23).
The second stage,
decision, involves computing the cost associated with attaining a reward. Depressed patients often report decision-making problems (
16,
24), sometimes seen by others as “lack of initiative.” These complaints best map onto this second stage of reward processing and in translational terms correspond to the decision part of an experiment, when a subject chooses between available options, for example, in a gambling task.
The third stage is
action, during which effort is expended for a rewarding stimulus to be approached or a punisher to be avoided. Fatigue and low energy, commonly reported in patients with other depressive symptoms (
16,
24), map onto this action component. In translational terms, this corresponds to a part of the experiment where a subject performs an action, such as a lever or button press, providing a quantification of task-related effort.
The final stage involves
experience, which encompasses the consummation of a reward and the feelings that may be associated with it. This phase also entails the consolidation of this experience in memory, which may be accessed for future reward processing. Consummatory anhedonia, the lack of enjoyment from activities that used to be pleasant, best maps onto this phase. Translationally, this corresponds to a subject being faced with either a win or a loss outcome within a task, such as occurs in the monetary incentive delay task. In EEG studies, this is measured in terms of the FRN potential, or its reverse reward positivity (
11), which occurs after feedback and is typically recorded at central to frontal-central regions of the scalp. FRN and reward positivity are the contrast of neural response to feedback of loss minus gain, and gain minus loss, respectively.
Reward processing involves many distinct components. One particularly key component of reward processing involves learning, whereby organisms update associated values attributed to objects and actions in their environment. Reward-related learning typically occurs through reward prediction errors, striatal dopamine-encoded signals that indicate the difference between anticipated and experienced reward (
19). Such learning influences subsequent decision making and updates anticipation. In that sense, all the phases depicted in the model are part of reward-related learning.
Blunting of reward responses has been observed in major depression in adolescents, but it remains unclear whether the magnitude of this signal reduction varies across development. The sharp increase in depression incidence during adolescence (
25) highlights the importance of examining this issue.
Discussion
This work links depression to aberrant reward processing. In particular, functional imaging and electrophysiological findings converge to show a blunted neural response to reward, and this effect may be more pronounced in individuals under age 18.
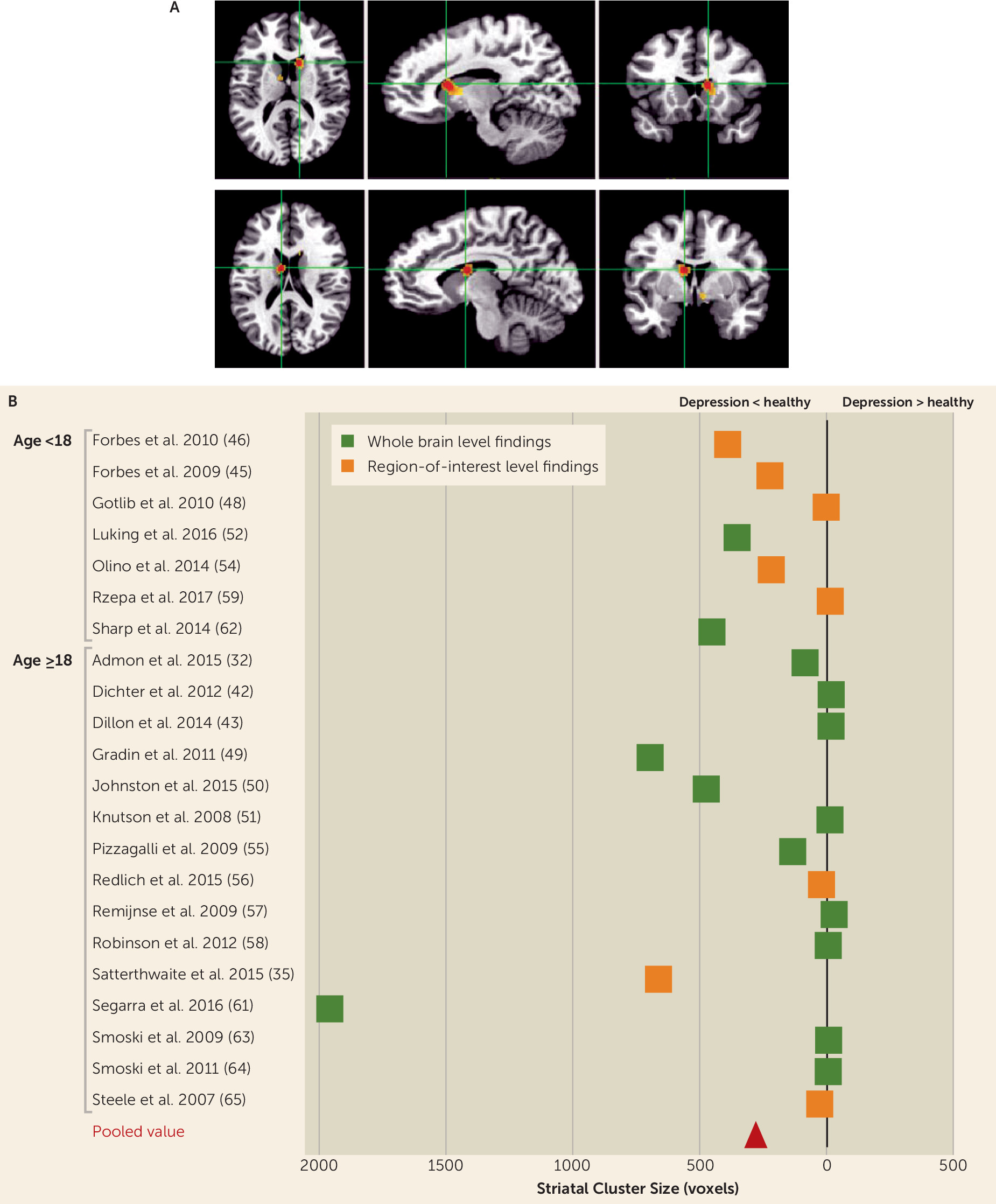
Our meta-analysis of fMRI studies found decreased striatal activity in subjects with depression compared with healthy volunteers during reward feedback. This finding is in keeping with the meta-analysis of Zhang et al. (
6), although only 25% of studies included in our meta-analysis overlapped with those of Zhang et al., with the addition of several (N=15) new studies published since then. These findings cannot be attributed to localization bias, as they also occur in non-region-of-interest studies. We also found decreased anticipation activity in depressed subjects when we lowered the statistical threshold or added region-of-interest studies. Reward anticipation and feedback are distinguished conceptually; it has been suggested (
86) that dopaminergic neurons are primarily associated with anticipation of reward (
87). By contrast, opioid neurons are associated with consummation of reward and therefore the feedback phase. The fMRI measures do not allow distinctions at the neurotransmitter level, and macroscopic anatomical overlap should not be taken to imply mechanistic overlap. There were no significant results for fMRI contrasts of loss. Because of the small number of studies, we combined loss anticipation and feedback (
88), and this may have diluted effects.
A previous meta-analysis (
10) found no differences overall between healthy and depressed subjects, but that analysis focused on a broad range of emotional and learning-related responses, rather than on strictly defined reward processing, as our study did. Moreover, the authors excluded studies with participants under age 18, whereas our study used a developmental approach including all ages and comparing adolescence with adulthood. Furthermore, we found evidence from longitudinal studies that aberrations in reward processing were predictive of new-onset depression (
33) and increased the risk for depression (
81). Interestingly, a recent connectivity study (
89) demonstrated that increased connectivity of the ventral striatum predicts depression, in keeping with striatal aberrations in this disorder.
Our fMRI findings fit with predictions from animal work (
90) on the centrality of the striatum in reward processing. It is notable that the peak of activity difference between healthy volunteers and depressed subjects is in the caudate, rather than in the nucleus accumbens, a key part of the circuitry associated with reward processing (
91). Indeed, there is substantial cytoarchitectural overlap between the accumbens and ventromedial parts of the caudate and putamen (
92), and they are collectively designated as the ventral striatum (
92,
93). The striatum receives rich input from various cortical areas, including the ventromedial prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex, as well as the amygdala and hippocampus (
93,
94). This input is integrated and then translated into action via neighboring areas in the basal ganglia.
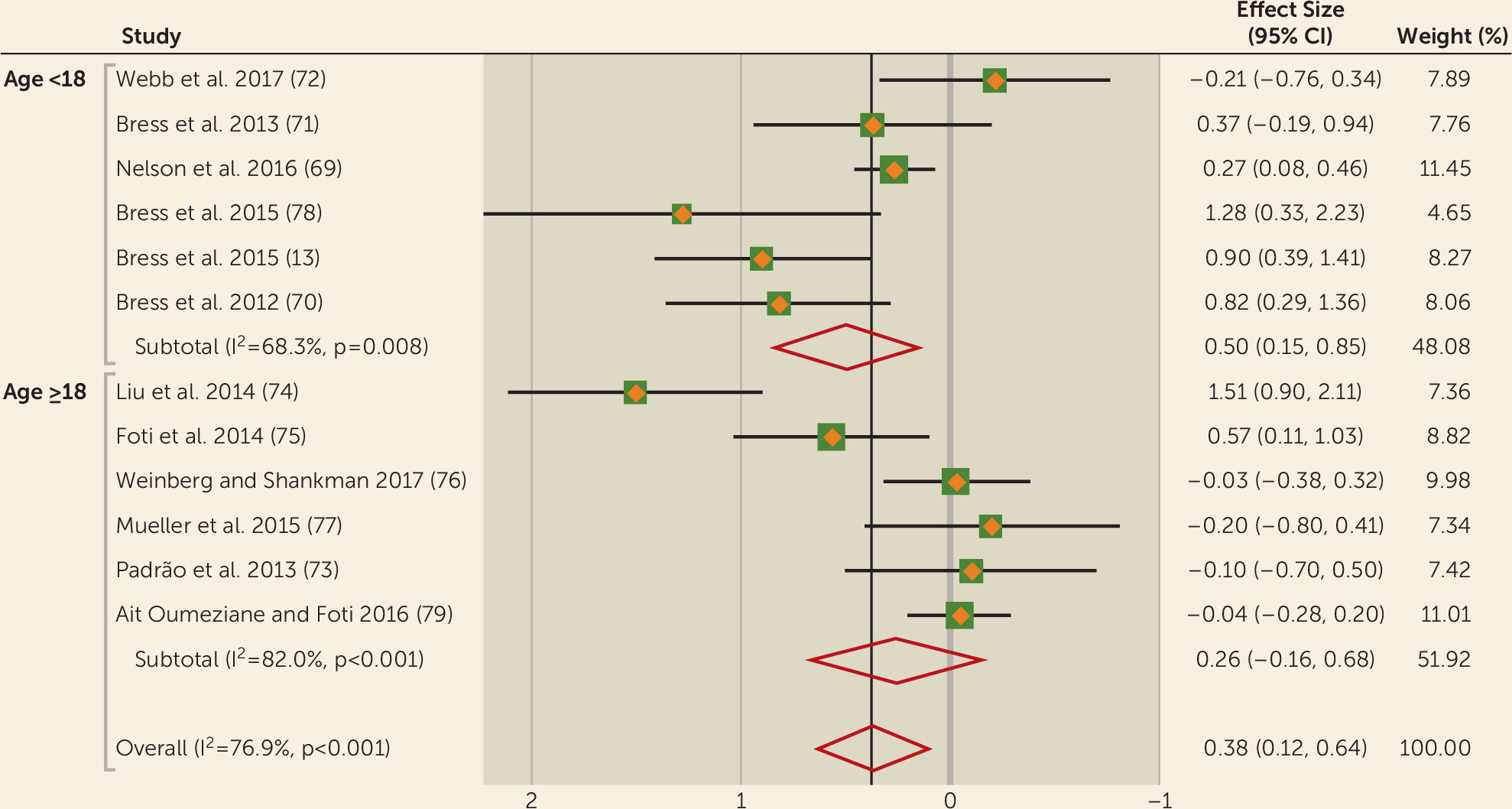
The overall association between FRN and depression yielded a significant effect size of 0.38 in the random-effects analysis. When we stratified our samples into subjects under age 18 and age 18 and older, significance was only found in youth depression but not adult depression, although the moderation statistic was not significant. We also noted evidence that blunted FRN is a predictor of future depression onset (
71). Longitudinal effects were observed in adolescents only, as no studies examined this association in adults. Taken together, and in line with the fMRI studies, these results suggest a decreased brain sensitivity to anticipating and consuming rewards in depression. While fMRI studies suggest that this deficit involves the striatum, the source of the FRN is still debated; however, it may partially reflect striatal signals (
95,
96) or the indirect influence of striatal signals on other neural regions (
97,
98). It is worth speculating about the fact that there was a lower heterogeneity in the younger than the older subsamples. The younger subsamples were more likely to be community based, were narrower in age range, and had lower levels of medication, whereas the older sample was more diverse in terms of demographic variables. Medication was not consistently reported among the studies, and therefore we could not assess its effects on the outcomes. It should also be noted that the younger samples contained more females than did the older samples, which may have influenced the results.
The reward system is known to undergo transition during the adolescent period, with changes indexed by FRN (
99,
100) and BOLD signal (
14). More studies, particularly longitudinal studies of depressed individuals that span adolescence and adulthood, will be needed to understand the interaction between development and depression.
When considering these findings, several conceptual and empirical challenges need to be considered. First, postulating depression to be a generalized inability to anticipate or perceive pleasure (or avoid pain) may be overly simplistic. Depressed individuals can still crave rewards, as evidenced by the increased levels of drug and alcohol dependency in depression (
101). Anhedonia is a core feature of depression closely linked to reward processing (
102). Unfortunately, few studies have included measures of anhedonia to quantify the degree to which reward system dysfunction is moderated by anhedonia level. Moreover, depression studies are needed that combine the high temporal precision of EEG or magnetoencephalography with the spatial precision of fMRI.
Second, few studies have demonstrated aberrations in depression that span the three levels of explanation: brain circuitry, task behavior, and clinical symptoms. Indeed, many of the tasks addressing reward processing, notably the monetary incentive delay task, are less suited to capturing behavioral effects and reward experience (
103). Developing tasks to overcome such shortcomings will be important. It will also be important to explore the interplay between reward and cognitions relevant to depression, such as executive control (
104).
Third, future studies should go beyond typical case-control designs to include comparisons of reward processing between subjects with depression and other morbid groups. We found very few studies that directly compared reward processing in depression alongside other disorders. Two studies that compared reward processing across alcohol dependence, schizophrenia, depression, or bipolar disorder found that decreased striatal activity was correlated with depressive symptoms (
35,
36).
Fourth, there were surprisingly few experimental studies embedded in treatment studies. Deep brain stimulation is the most direct way of testing this, although it is the most ethically challenging. Promising initial results of deep brain stimulation of the ventral striatum (
105,
106) did not replicate in controlled studies (
107). While some pharmacological (
31,
108,
109) or psychological (
110) interventions show promise in probing reward signal, they do not yet demonstrate that affecting reward modulates depressive symptoms.
Fifth, the extant studies in the literature allowed us to pool results for only two of the four postulated components of reward processing that we outlined above. Clearly, more research is needed to understand the functioning of the other component processes at the neural level in depression. Our review also could not address directly the important issue of reward learning (
8), as there were not enough imaging or EEG studies of reward learning in depression that fit our criteria.
Sixth, we found no evidence of publication bias for the EEG studies but cannot exclude the possibility that the nonreporting of null results biased the fMRI findings.
Overall, these findings demonstrate consistent reward processing aberrations in depression, expressed as blunted striatal fMRI and FRN signals, during reward feedback. These aberrations, which potentially underlie the pathogenesis of depression, may have important implications for the development of new treatments.