Despite decades of medical research for what remains a substantial public health problem (
1), there are no medications approved by the U.S. Food and Drug Administration for the treatment of cocaine use disorder. Standard behavioral treatments, meanwhile, are beset by high failure rates. A line of pharmacotherapy research that holds promise is modulation of glutamate neurotransmission (
2,
3). The
N-methyl-
d-aspartate receptor (NMDAR) is the predominant glutamate receptor involved in learned behavior (
2), and NMDAR modulators have been shown to disrupt the reinforcing effects of cocaine in animals (
4,
5), although comparable modulators, such as memantine, have yet to show efficacy in humans (
6–
8).
The dissociative anesthetic ketamine may represent an exception. Ketamine has been widely demonstrated to have robust antidepressant properties when administered at subanesthetic doses (
9), and its psychiatric mechanisms are hypothesized to extend beyond NMDAR modulation to include downstream effects on other neurotransmitter systems and on prefrontal synaptogenesis (
9). These mechanisms may also be relevant to treatment of cocaine use disorder (
2,
10–
12); a single subanesthetic infusion of ketamine has been shown to exhibit substantial effects in cocaine-dependent research volunteers, including heightening motivation to quit cocaine use, reducing craving, and decreasing cocaine use in a laboratory setting (
10,
11).
Mindfulness-based relapse prevention (MBRP), a manualized treatment that has demonstrated modest efficacy for substance use disorders (
13,
14), may be especially well suited as a behavioral counterpart to ketamine. In addition to training individuals in cognitive-behavioral strategies, MBRP incorporates practices to cultivate “mindfulness,” an attitude of deliberate, present-centered awareness, coupled with a suspension of behavioral reactivity and of cognitive associations, judgments, and distortions (
13–
15). Interestingly, mindfulness practices are thought to provide benefit via neural mechanisms similar to those attributed to subanesthetic ketamine (
4), including the regulation of mesolimbic functioning (
16), the promotion of prefrontal neural plasticity and synaptogenesis (
17,
18), and sustained modulation of default-mode network hyperconnectivity (
4,
19–
23).
In light of this overlap in neural and behavioral effects, it is possible that ketamine and MBRP may work together to promote sustained abstinence. The primary purpose of this randomized trial was to investigate whether a single subanesthetic ketamine infusion (0.5 mg/kg i.v. over 40 minutes) promotes abstinence relative to the active control midazolam in cocaine-dependent adults initiating MBRP. Other aims included assessing the effect of ketamine on craving and on time to relapse.
Methods
Fifty-five individuals seeking treatment for cocaine dependence were enrolled and randomly assigned to receive a 40-minute intravenous infusion of ketamine (0.5 mg/kg) or of midazolam (0.025 mg/kg) in a 5-week trial, which started in September 2011 and concluded in December 2016. Participants were hospitalized for 5 days on a psychiatric research unit, during which time they initiated MBRP and received daily sessions of the behavioral treatment through day 5. On day 2 they received an infusion, and on day 5 they were discharged. Participants returned twice weekly for 4 weeks for MBRP sessions, monitoring and measures (including urine toxicology), and physician visits. At the end of the trial, during week 5, they were provided referrals to other treatments. A follow-up interview was conducted by telephone at 6 months.
Participants
During screening, participants met with research staff who administered the Mini International Neuropsychiatric Interview (
24) and with a physician for a medical and psychiatric evaluation that included serum collection, additional diagnostic tests, and completion of the Hamilton Depression Rating Scale (
25) and the Dissociative Experiences Scale (
26). Applicants were considered eligible if they were medically healthy treatment-seeking adults under age 70 who met DSM-IV criteria for cocaine dependence, with no psychiatric comorbidity, and who additionally met minimum cocaine use criteria over the 4-week period preceding screening (one occasion of cocaine use per week at >$200; two occasions of cocaine use per week at >$40 each time; or three or more occasions of cocaine use a week at any amount; and urine toxicology indicative of cocaine use on at least one occasion during screening). Individuals with a history of psychotic or dissociative symptoms and with current depressive or anxiety symptoms indicative of a DSM-IV disorder (
27) were excluded, as were individuals with benzodiazepine or opioid use disorder. The study was approved by the New York State Psychiatric Institute institutional review board, and all participants provided written informed consent.
Setting
All procedures and outpatient visits occurred at the New York State Psychiatric Institute (NYSPI) on the Columbia University Medical Center campus. The inpatient stay occurred in the NYSPI clinical research unit, which is staffed by a team of psychiatrists, social workers, nurses, and technicians. All staff in both settings were blinded to participants’ treatment assignment.
Infusion Procedures
On day 2 of the inpatient phase, participants were randomly assigned in a 1:1 ratio to either the ketamine condition or the midazolam control condition by a statistician not otherwise involved with study procedures using randomly sized blocks. Blinded staff were involved with the infusion administration.
The inpatient setting ensured that participants abstained from cocaine or other intoxicants for at least 24 hours before the infusion. Additionally, they did not eat anything after midnight the night before, so as to reduce the risk of nausea and aspiration. Participants were not told that they would receive either ketamine or midazolam; instead, they were told that they might receive any of several medications: amantadine, buspirone,
d-cycloserine, ketamine, memantine, midazolam, or saline. This blinding procedure was intended to disguise what drug was given so as to minimize expectancy effects (
10,
11). Similarly, it aimed to minimize the risk that participants would clearly identify the medications that were administered.
Ketamine hydrochloride (0.50 mg/kg) or active control (midazolam 0.025 mg/kg) was prepared in saline and packaged by the NYSPI pharmacy for a slow-drip 40-minute intravenous infusion. Infusions were administered at approximately 11 a.m. on day 2. The dose of ketamine was selected on the basis of published reports suggesting that it was well tolerated and potentially effective for a variety of psychiatric disorders (
9). Midazolam was chosen as the active control because it produces a mild change in consciousness, without any known persistent (>8 hours) effect on cocaine dependence or mental state (
11,
28). Blood pressure, heart rate, and blood oxygen saturation were continuously monitored. Medical coverage was provided during and up to 2 hours after the infusion; a psychiatrist provided a brief safety and psychiatric evaluation at the end of the monitoring period.
As in our previous studies (
10,
11), we provided relaxation and breathing exercises to prepare participants; they were also guided through these practices if any discomfort or anxiety emerged during infusions. Specifically, participants were instructed in the “body scan” (mindfully attending to sensations throughout the body while supine), as described in the MBRP manual (
29). The Clinician-Administered Dissociative States Scale (CADSS) (
30) was administered at the conclusion of the infusion by a trained research assistant. Participants also completed various assessments pertaining to subjective effects. A session of MBRP was provided that afternoon, approximately 2 hours after the infusion.
MBRP and Outpatient Visits
Participants engaged in MBRP delivered by trained master’s-level staff, adapted to the individual setting and shortened from 8 weeks to 5 weeks in collaboration with one of its lead authors (Sarah Bowen, Ph.D.). Four sessions of MBRP were administered on days 2 through 5 during the inpatient phase, with the remaining sessions provided weekly. The manual consists of a progression of practices and discussion points that are intended to cultivate mindfulness (e.g., breathing and visualization exercises), to assist in integrating mindfulness into daily life, and to promote application of mindfulness to addiction-related vulnerabilities, such as reactivity in high-risk situations (
29). Providing half of the sessions during the inpatient phase was intended to deliver high-density behavioral treatment during the period when ketamine is hypothesized to exhibit peak psychiatric, and possibly anti-addiction, efficacy (up to 72 hours after infusion), enhancing the possibility of sustained behavioral modification (
9–
11). Sessions were recorded and overseen by a senior therapist (K.M.C.) or by the principal investigator (E.D.) to ensure fidelity and minimize intertherapist discrepancies.
Participants returned to clinic twice weekly during weeks 2 through 5, once for an MBRP session and once for a visit with a study physician. Measures related to cocaine-related vulnerabilities, such as craving (assessed using a visual analogue scale), mindfulness (the Five-Facet Mindfulness Questionnaire), and stress sensitivity (the Perceived Stress Scale), were collected at each visit, as well as urine toxicology (12-panel dipstick, including ketamine) and self-reported drug use (cocaine and other substances, including alcohol, benzodiazepines, and ketamine), using the timeline followback method (
31). Physicians were advised to examine participants for adverse events unique to the study medications, such as persistent psychoactive effects and the emergence of ketamine or benzodiazepine misuse.
Participants were compensated with $30 during screening, $40 for completion of the inpatient phase, and $5 at each outpatient visit to defray the costs of travel.
Statistical Analysis
Baseline demographic characteristics, side effects, and adverse events were tabulated and summarized using means and standard error (or median and interquartile range for nonnormally distributed outcomes). Differences between groups on dissociative symptoms (as assessed with the CADSS) were analyzed using the Wilcoxon rank-sum test. The between-group difference in change from baseline to postinfusion assessment on the Dissociative Experiences Scale and in peak systolic blood pressure were also analyzed using Wilcoxon nonparametric rank-sum tests.
The primary outcome was 2 weeks of end-of-study abstinence, confirmed by urine toxicology; if participants denied use between visits and yet provided urine samples indicative of recent cocaine use, they were designated as not abstinent for that self-report period. The proposed sample size was 60 individuals, although the research was stopped after an interim analysis (N=55) requested by the National Institute on Drug Abuse demonstrated a difference that would remain significant even if the trial finished according to a “worst-case” algorithm. Logistic regression was used to analyze the effect of treatment on the primary outcome in the interim and present analyses. Time to relapse (defined as first use or dropout after the inpatient phase) was analyzed using the Cox proportional hazard model.
The secondary outcomes of weekly cocaine use (during weeks 2 through 5) and weekly craving scores (during weeks 1–5) were analyzed using longitudinal mixed-effect models with the effect of study week, treatment, study week-by-treatment two-way interaction, and the corresponding outcome measured at baseline (when appropriate). A random intercept was used to account for the between-subject variances. The logit link function was used to model binary cocaine use, and the log link function was used to model craving scores to account for their nonnormal distribution. Consistent with previous cocaine use disorder trials, route of use was included as a covariate in all analyses. Analyses were performed using SAS, version 9.4, with all tests two-sided at a 5% level of significance.
Results
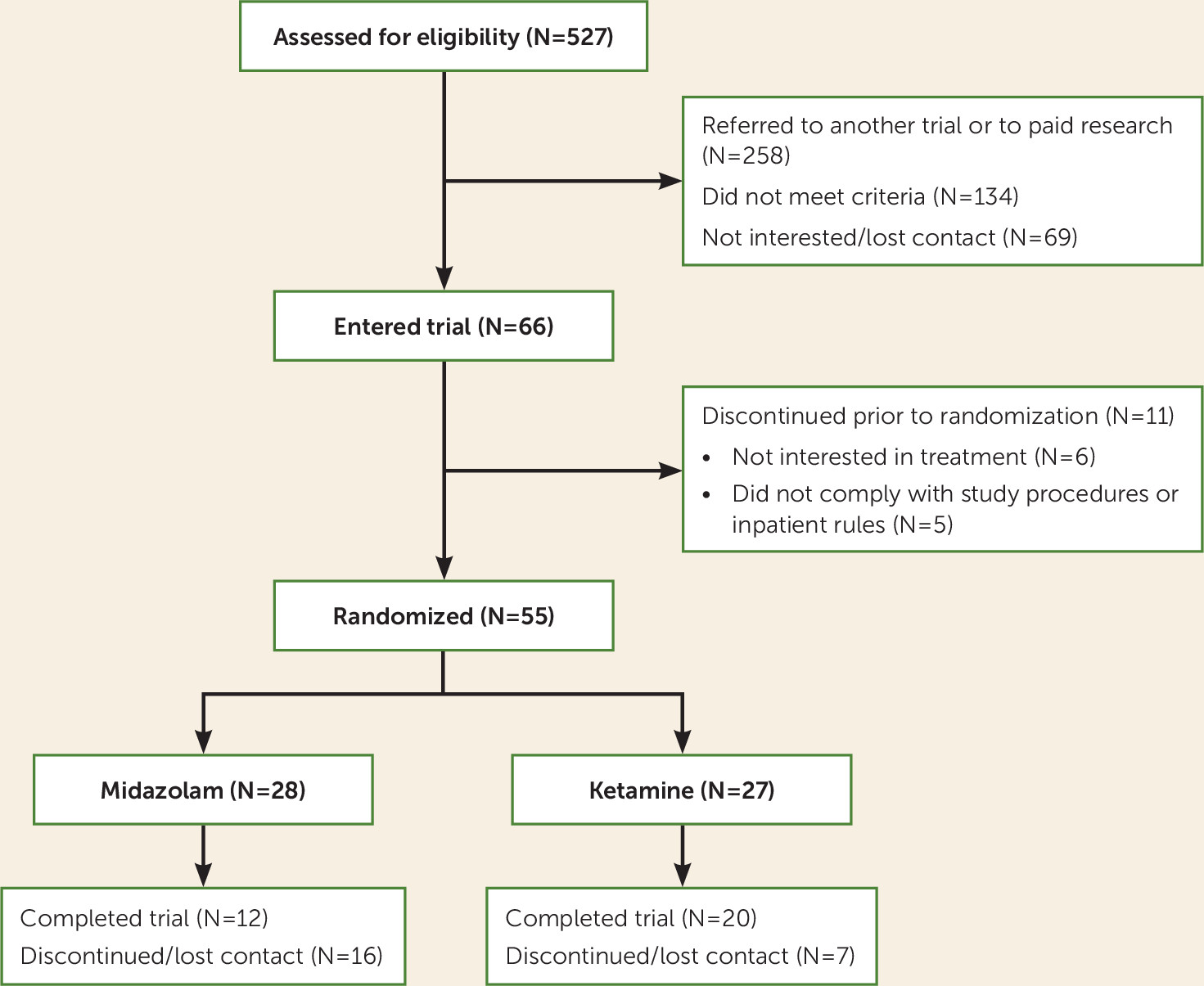
Fifty-five individuals underwent randomization (
Figure 1). The median baseline cocaine use (in dollars per day) was $32.86 in the ketamine group and $36.43 in the midazolam group. The proportion of freebase (smoked cocaine) users was 71.4% in the midazolam group and 55.6% in the ketamine group (see
Table 1 for other participant characteristics).
Infusions
Treatment with ketamine was significantly associated with higher ratings of acute dissociation on the CADSS; the median score was 22 (interquartile range [IQR]=13, 34) in the ketamine group and 7 (IQR=4, 10) in the midazolam group (χ2=6.25, df=1, p<0.001). All psychoactive effects resolved within 30 minutes after infusion. There was no persistent dissociation (as assessed by the Dissociative Experiences Scale after infusion) in either the ketamine group or the midazolam group (median change, 0.0 [IQR=−2.1, 1.4] and −1.0 [IQR=−4.8, 9.2], respectively; χ2=0.06, df=1, p=0.82). Systolic blood pressure increased significantly during ketamine infusions compared with midazolam (median change, 19.5 mmHg [IQR=17.0, 30.0] and 8.0 mmHg [IQR=4.0, 12.5], respectively; χ2=20.14, df=1, p<0.001). The only reported adverse effect was mild sedation lasting no longer than 12 hours (reported in seven participants in the midazolam group and three in the ketamine group). There were no instances, in either group, of persistent psychiatric disturbances, clinical worsening, increased drug use, or emergence of new drug misuse (ketamine, opioids, or benzodiazepines), as assessed by self-report, urine toxicology, and psychiatric assessments.
Abstinence
The proportion of participants in the ketamine group with urine-test-confirmed abstinence over the last 2 weeks of the trial was 48.2% (13/27), compared with 10.7% (3/28) in the midazolam group. The odds of end-of-study abstinence in the ketamine group was 6 times that in the midazolam group (odds ratio=5.7, 95% CI=1.3, 25.1; χ2=5.34, df=1, p=0.02) when route of use was controlled for. There was no significant difference between groups in alcohol, cannabis, or tobacco use.
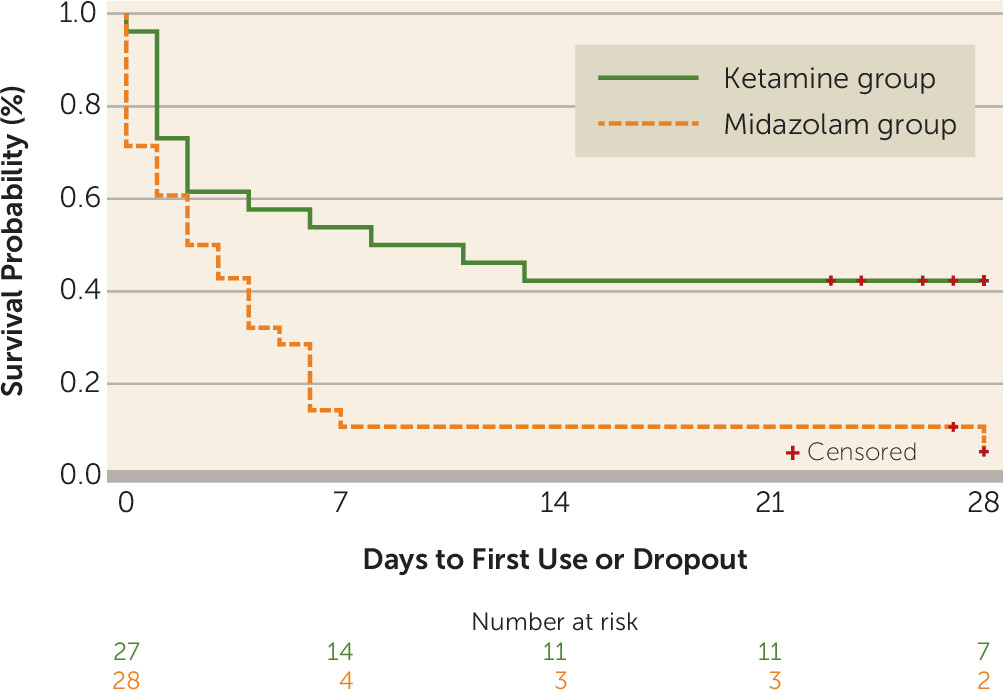
Time to Relapse
Among participants in the ketamine group, 57.7% (15/27) went on to use cocaine or drop out, compared with 92.9% (26/28) of those in the midazolam group. The test of the proportional hazards assumption was not significant (χ
2=3.86, df=2, p=0.15), suggesting that it was not violated. Cox regression shows that the ketamine group was 53% less likely (hazard ratio=0.47, 95% CI=0.24, 0.92; χ
2=4.78, df=1, p=0.03) to relapse (drop out or use cocaine) compared with the midazolam group when route of use was controlled for (
Figure 2).
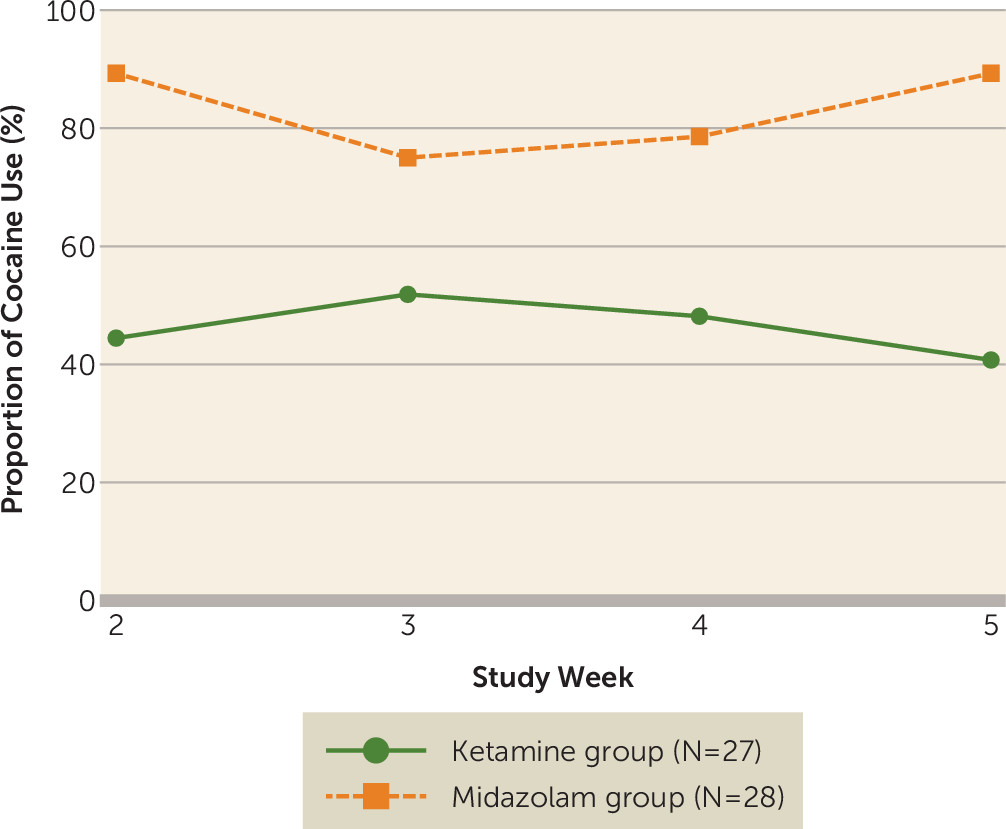
Cocaine Use
The effect of the two-way interaction between time and treatment on cocaine use was not significant (t=−0.43, df=163, p=0.67), and it was omitted from the final longitudinal mixed-effects model. According to the final main-effects model, the odds of cocaine use in the midazolam group were 7.8 times the odds in the ketamine group (odds ratio=7.8; 95% CI=1.5, 39.9; t=2.50, df=164, p=0.01) when route of use was controlled for. There was no change in drug use over time in either group (t=−0.29 df=164, p=0.77), suggesting that participants in the ketamine group maintained their early improvements over the course of the trial (
Figure 3).
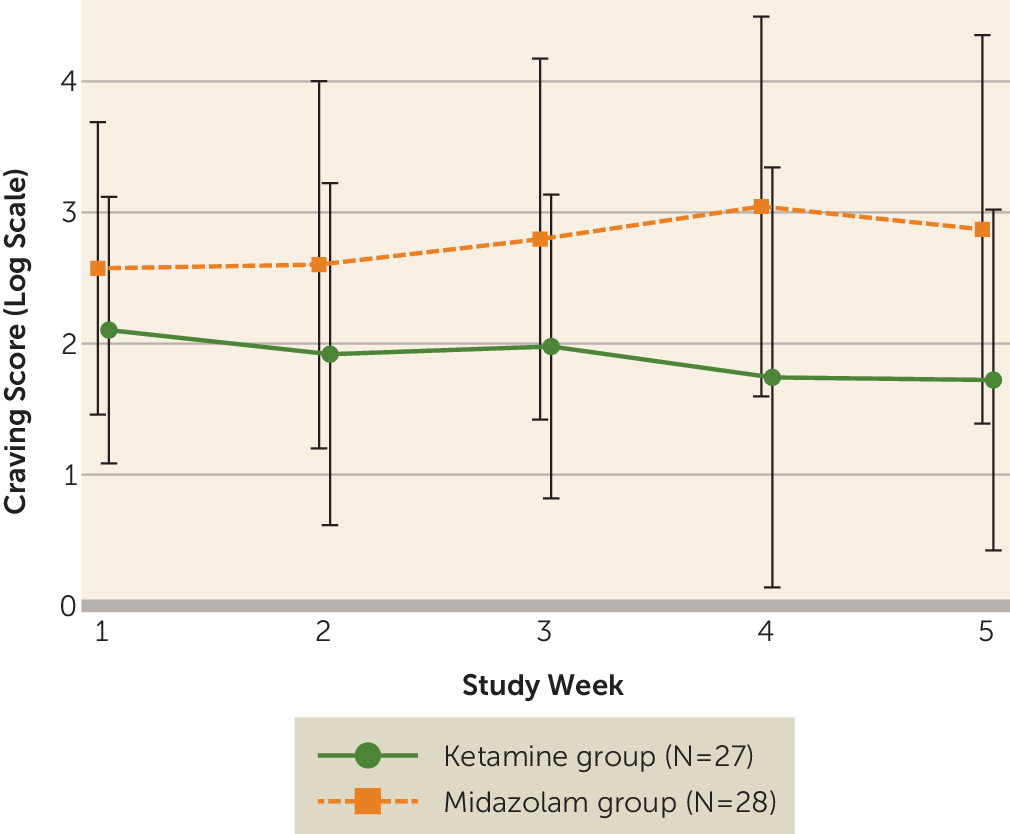
Craving and Other Measures
The effect of the two-way interaction between time and treatment on craving scores was not significant (t=−1.96, df=99, p=0.053), and it was omitted from the final model. In the final main-effects model, craving scores were 58.1% lower in the ketamine group than the midazolam group (t=−2.57, df=100, p=0.01; 95% CI=18.6, 78.6) when route of use was controlled for, and there was no significant change in craving scores over time for either group (t=−1.59, df=100, p=0.11). This suggests that early improvements in craving in the ketamine group were sustained over time (
Figure 4). There were no differences between groups over time in measures of stress sensitivity, as assessed with the Perceived Stress Scale, and of mindfulness, as assessed with the Five-Facet Mindfulness Questionnaire.
Follow-Up
At the 6-month telephone follow-up interview, 44% (12/27) of the participants in the ketamine group reported that they were abstinent, compared with none of the participants in the midazolam group. The proportion of individuals who were abstinent was significantly associated with treatment group (χ2=15.92, df=1, p<0.001).
Discussion
This is, to our knowledge, the first clinical trial to investigate a subanesthetic dose of ketamine in cocaine-dependent individuals, as well as the first to investigate a single intravenous infusion in the context of behavioral treatment. We found that ketamine was well tolerated and promoted abstinence. We also found that, compared with midazolam, ketamine was associated with a lower likelihood of cocaine use, lower levels of cocaine craving, and longer time to relapse. A single ketamine infusion also led to a greater proportion of individuals remaining cocaine free at the 6-month telephone follow-up interview. These results suggest that ketamine, in combination with mindfulness-based behavioral modification, may be an effective pharmacotherapy for cocaine use disorder.
Previous studies with ketamine in cocaine-dependent individuals were laboratory-based trials investigating the subacute effects of ketamine (24–48 hours after infusion) on cocaine-related vulnerabilities in the absence of any behavioral treatment (
10,
11). Any improvements that were observed, such as reductions in use and craving, were typically transient, subsiding after several days (
11). These preclinical data indicated that the benefits of ketamine are generally brief and would need to be extended in some manner to have a clinical impact.
One strategy for sustaining these effects is to embed ketamine infusions into an addiction-oriented behavioral framework. Such a combination might leverage the impact of ketamine into longer-lasting changes, especially in perspective, decision making, and relapse prevention skills (
12). Early trials evaluating “ketamine psychedelic therapy” also employed an integrated approach, with intramuscular ketamine combined with existentially oriented psychotherapy to treat alcohol and opioid use disorders (
32,
33). We designated MBRP as the behavioral platform for ketamine because the two interventions share hypothesized neural mechanisms and have comparable effects on dependence-related vulnerabilities, such as reactivity and craving. Also, the psychoactive effects of ketamine, specifically its mystical-type phenomena, suggest some similarities with the meditative experience (
34) and might work to provide an experiential stepping-stone toward deepening mindfulness training.
While this trial was not designed to evaluate synergy between ketamine and MBRP, it served to evaluate whether ketamine provides additional benefit to individuals engaged in this behavioral treatment. The group receiving ketamine demonstrated higher rates of retention, consistent with the previous finding that ketamine enhances motivation to change drug use and supporting the hypothesis that it might serve to facilitate engagement with behavioral treatment (
10). MBRP also appeared to be helpful in carrying forward the effects of ketamine beyond what has been observed in previous research (
10,
11), with participants maintaining abstinence and experiencing sustained craving reduction through several weeks. It is not possible, however, to conclude whether MBRP was necessary for sustaining the benefits of ketamine because our study did not include a treatment group that received medication without MBRP; it is conceivable, although unlikely based on previous work (
10,
11), that ketamine might have led to these results in the absence of any behavioral treatment. Similarly, another psychosocial framework might have been just as helpful a behavioral counterpart, as we did not compare different behavioral treatment and medication combinations. This trial suggests, in any case, that the effects of ketamine might be sustained when integrated into behavioral treatment; future research can examine this hypothesis more rigorously.
Further clarification of the therapeutic mechanisms of ketamine can assist in our understanding of how best to harness its clinical potential. A range of pathways have been hypothesized to account for the antidepressant efficacy of ketamine, from receptor-specific changes to neurotrophic, modulatory, and even psychological mechanisms (
9,
22,
35–
37). Some of these mechanisms are relevant to substance use disorders as well and suggest innovative strategies for enhancing or replacing ketamine. For example, preclinical research indicates that an infusion of brain-derived neurotrophic factor (BDNF), which has also been shown to be elaborated by ketamine (
9), into the brains of rodents disrupts cocaine self-administration (
38). This suggests that pharmacotherapy supplementing the BDNF-related effects of ketamine may serve to sustain efficacy.
A recent small study suggested that opioid-related mechanisms may be involved in the antidepressant effects of ketamine (
39). Although these findings are preliminary, they call attention to why ketamine might be abused and to the importance of appropriately addressing this abuse liability in clinical settings. As in other clinical procedures involving substances of abuse liability (e.g., provision of postoperative opioids), there are various safeguards that work to minimize these risks, such as careful patient selection, preparation, monitoring, and postintervention support (
9–
12). These safeguards were incorporated into the present study as well as into the studies of ketamine in depressive and anxiety disorders; there has been no incidence of ketamine misuse in any of the research to date, suggesting that this risk can be effectively managed even when this agent is given to substance users (
10–
12,
40). The behavioral and mindfulness-based framework may have also worked to guide participants to engage with the infusions in a therapeutic manner, cultivating a perspective that is not grasping of any particular sensation or experience, but that is accepting, deliberate, and nonreactive.
This trial has several limitations. The sample was relatively small, and there were minor discrepancies between treatment arms in demographic characteristics and preferred route of use. Future trials with larger samples and with randomization stratified by route of use should work to address this limitation. Furthermore, a larger sample may provide sufficient power to evaluate the impact of ketamine on the use of other substances that may be related to cocaine, such as tobacco and alcohol. Second, the study procedures were highly monitored, including an inpatient phase. Inpatient treatment of cocaine use disorders is uncommon, and the level of monitoring provided is unusual in standard practice. Third, the sample was relatively homogeneous, with a majority of participants African American and male, and with minimal psychiatric comorbidity. While the selection of patients with no comorbidities might hamper the extension of our findings to a more psychiatrically complicated population, it is likely that cocaine-dependent individuals with depression or anxiety would respond even more robustly to ketamine given evidence supporting its psychiatric efficacy (
9). Future trials can examine the effect of ketamine on individuals with drug dependence and psychiatric comorbidity, as well as examine sex differences in the response to ketamine.
Another limitation pertains to the psychoactive effects of ketamine, which can be pronounced and easily identifiable in some cases. Although staff members involved in infusion procedures were not involved in subsequent data collection, and the primary outcome was unlikely to be affected by evaluator bias (e.g., drug use in the natural ecology), we did not test the integrity of the blind by ascertaining what medication participants or staff believed was administered during infusions. The minor deception we enforced to further protect the blind and minimize expectancy effects (i.e., informing participants they may get any of several medications) constitutes a limitation as well. This deception would not be enforced in clinical settings, and it raises questions about how abuse liability would be addressed when individuals with cocaine use disorder know they will be treated with ketamine.
Despite these limitations, these data represent a promising step in understanding the clinical role of ketamine in addiction treatment. Ketamine was effective at providing individuals already engaged in mindfulness-based behavioral modification with significantly greater odds of maintaining abstinence, substantial protection from relapse and craving, and lower likelihood of cocaine use. These sustained benefits, in some cases lasting several months, suggest the potential of ketamine for effecting long-term behavioral changes. Moreover, the integrated treatment framework was well tolerated, and MBRP appeared to enhance certain ketamine-related benefits, such as reductions in cocaine craving scores. Further research is needed to replicate these results in a larger sample, as well as to clarify mechanisms, examine more rigorously the hypothesis of synergy between ketamine and behavioral treatments, and evaluate emerging pharmacotherapies comparable to ketamine.