Posttraumatic stress disorder (PTSD) is a DSM-5 psychiatric syndrome that develops after exposure to a traumatic event (
1). PTSD is a heterogeneous disorder often characterized by an assortment of hyperarousal, reexperiencing, and avoidance/numbing symptoms, as well as negative alterations in cognition and mood (
1). Epidemiological reports indicate that up to 70% of the general population will experience a traumatic event during their lifetime, and 7% of those individuals will go on to develop PTSD (
2). Rates of trauma exposure and lifetime PTSD are higher in military samples (
3) and in urban environments (
4). Because the prevalence of PTSD and the adverse psychological and economic consequences of PTSD continue to climb, it is imperative that prospective biomarkers be characterized so that individuals at risk for developing PTSD in the aftermath of trauma exposure can be identified. Early identification will allow earlier treatment with psychological and pharmacological interventions that have been shown to be effective in attenuating PTSD development (
5,
6).
One biological system that may confer individual risk for development of PTSD is the immune system (
7). Increased basal concentrations of proinflammatory cytokines, including interleukin (IL) 1β, IL-6, and interferon-γ (IFNγ), have consistently been found to be associated with PTSD (
8). Higher concentrations of chemokines and immune system growth factors have also been associated with trauma exposure (
9) and chronic PTSD (
10,
11). Additionally, resilience to traumatic events (
12) and PTSD are associated with higher concentrations of anti-inflammatory cytokines, such as IL-10 (
11,
13). Basic and translational data show that proinflammatory cytokines can directly affect the CNS, as demonstrated by measures of neurochemistry (
14) and brain circuitry implicated in the pathophysiology of PTSD, including the amygdala (
15,
16) and the prefrontal cortex (
17), to facilitate the expression of the cardinal psychiatric symptoms of PTSD. While many studies have demonstrated an association between a proinflammatory state and PTSD, there is a paucity of evidence on whether inflammatory markers can serve as prospective risk factors for the development of PTSD.
To date, only one study has addressed the question of whether heightened systemic inflammation prior to trauma exposure is a risk factor for the development of PTSD in the aftermath of trauma (
18). That data, from the Marine Resiliency Study, showed that higher predeployment concentrations of C-reactive protein, a systemic inflammatory marker, increased risk for postdeployment development of PTSD in a predominantly male sample of marines (
18). Although the study was of interest in showing that heightened immune system activation confers individual risk for PTSD development, the difficulty in securing biological samples before trauma exposure in large numbers of individuals precludes the adaptability of such measures to assessment of prospective risk for PTSD. Thus, the present study was designed to characterize immune factors that predict chronic PTSD development in blood samples collected in the immediate aftermath of trauma exposure in an emergency department setting. Given preexisting data showing that a proinflammatory state is associated with greater PTSD symptom severity (
8), we hypothesized that higher concentrations of proinflammatory cytokines, including IL-1β, IL-6, tumor necrosis factor α (TNFα), and IFNγ, and higher concentrations of chemokines and growth factors in the acute posttraumatic period, would be associated with increased risk for development of chronic PTSD. Furthermore, we hypothesized that lower concentrations of anti-inflammatory cytokines would be associated with prospective development of PTSD.
Methods
Participants
Participants (N=505) between the ages of 18 and 65 years were recruited between June 2012 and January 2017 from the emergency department at Grady Memorial Hospital in Atlanta after experiencing a DSM-IV criterion A trauma within the past 24 hours (
19). Other inclusion criteria were ability to provide informed consent, ability to understand and speak English, provision of blood samples (obtained by emergency department care staff), and access to a telephone to allow contact for follow-up appointment scheduling. Individuals were excluded if they had a current or past history of mania, schizophrenia, or other psychoses; prominent suicidal ideation in the past month; intoxication, severe pain, active labor, or respiratory distress; anticipation of immediate surgery or admission to the intensive care unit; medical instability; or hemodynamic compromise.
Eligible trauma survivors were approached by trained evaluators in the emergency department after initial medical evaluation, appropriate laboratory testing, and medical clearance had occurred. After providing informed consent, participants underwent a bedside assessment that lasted 1.5 hours. Study participants returned for follow-up visits at 1, 3, 6, and 12 months for assessment of the development of PTSD symptoms. Participants were compensated $50 at each of the assessments for their participation.
All study procedures were reviewed and approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee. All study data were recorded and managed electronically using the REDCap platform (
20).
Measures
A standardized trauma interview was administered in the emergency department to collect sociodemographic data (e.g., sex, age, race, income, body mass index [BMI]) and characteristics of the presenting trauma exposure, including the type of trauma (interpersonal or noninterpersonal) (
21). The PTSD Symptom Scale (
22) was used to assess the development of PTSD symptoms at the 1-, 3-, 6-, and 12-month follow-ups, as described previously (
23); interrater reliability was 97%.
PTSD Symptom Trajectories
Latent growth mixture modeling was applied using Mplus, version 7, to determine how many distinct latent classes best describe the trajectories of PTSD symptom severity based on PTSD Symptom Scale scores in the study data set when available (only one assessment, 15.6%; two assessments, 13.3%; three or more assessments, 71.0%). To identify the best-fitting number of trajectory classes, we started with up to six possible classes and examined linear and quadratic slope to identify the best-fitting trajectory shape. We used a nested-model approach to test a progressive number of classes until the model fit indices no longer favored the addition of any more classes. Relevant criteria for determining the number of classes included the reduction in the Bayesian information criterion, sample-size-adjusted Bayesian information criterion, Akaike information criterion indices, and significance indicated by the Vuong-Lo-Mendell-Rubin likelihood and the bootstrap likelihood, together with parsimony and interpretability. Clarity of class specification was determined by entropy. Trajectory analyses were run on the sample of participants who had at least one follow-up assessment (N=274). Participants were assigned to one of the three identified trajectories (chronic, recovery, or resilient) on the basis of their most likely trajectory class membership (highest posterior probability).
Blood Sample Collection and Cytokine Measurements
Venous blood samples were collected in EDTA tubes in the emergency department after trauma exposure (mean time elapsed between trauma and blood sampling, 201 minutes [SEM=12.8]) by medical staff using standard techniques. Within 6 hours of collection, EDTA tubes were centrifuged at 4°C and the plasma was aliquoted into 500 μL samples and frozen at −80°C until time of assay. Twenty-seven cytokines, chemokines, and growth factors were quantified in plasma available from 274 participants with at least one follow-up assessment using a commercially available human multiplex assay (M500KCAF0Y, Bio-Rad, Hercules, Calif.) on a MAGPIX instrument (Luminex, Austin, Tex.).
Samples were run on four plates by an analyst who was blind to PTSD outcomes. Three samples were run on every plate to control for interplate variability (coefficient of variation, 0.7%−16%) and intraplate variability (coefficient of variation, 4.5%−11.2%). Assays were checked for quality control to fit the standard curve. One participant (assigned to the resilient class) was excluded as an outlier because of aberrantly high values across the board. Proinflammatory cytokines assessed were IL-1β, IL-6, TNFα, and IFNγ. Anti-inflammatory factors assayed were IL-10 and IL-1RA. Other cytokines (IL-5, IL-15, IL-2, IL-13, IL-4, IL-9, IL-12, and IL-17) were also assessed. Chemokines measured were eotaxin/CCL11, MIP-1α/CCL3, IP-10/CCL10, MCP-1/CCL2, IL-8, MIP-1β/CCL4, and RANTES/CCL5. Growth factors assayed were granulocyte macrophage-colony stimulating factor (GM-CSF), basic fibroblast growth factor (bFGF), IL-7, and platelet-derived growth factor BB (PDGF-BB). G-CSF and VEGF were not detected. Because of the low concentrations of IFNγ and the Bio-Rad kit’s lower assay sensitivity of 6.1 pg/mL, we confirmed the values in a subset of the participants (N=84) with a magnetic Luminex performance assay, human IFNγ high sensitivity (LHSCM285B, R&D Systems), to ensure that the low concentration values of IFNγ were reproducible with a different assay (data not shown).
Statistical Analysis
The data were analyzed using SPSS, version 24 (IBM, Armonk, N.Y.) and summarized as mean values and standard error of the mean. The alpha level was set at 0.05 for statistical significance. One-way analyses of variance and chi-square tests were used to assess baseline sociodemographic differences in PTSD trajectory class (chronic, recovery, and resilient). Multivariate analyses of covariance (MANCOVAs) were used to test our hypotheses that peritraumatic concentrations of proinflammatory and anti-inflammatory factors and cytokines, chemokines, and growth factors would be associated with increased risk for chronic PTSD symptom development, controlling for sex, BMI, time elapsed between trauma exposure and blood sampling, time of day of blood sampling, and incident and premorbid interpersonal trauma exposure.
Discussion
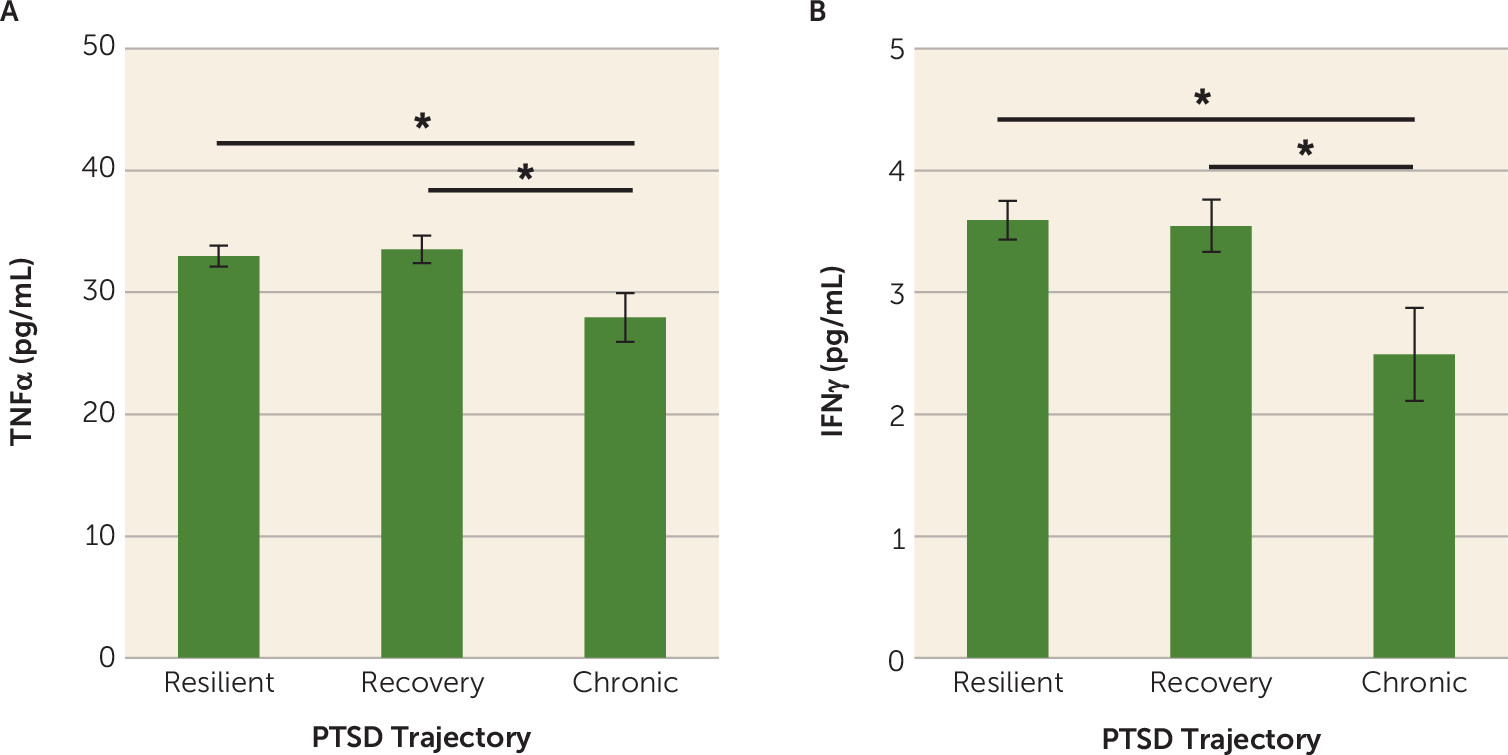
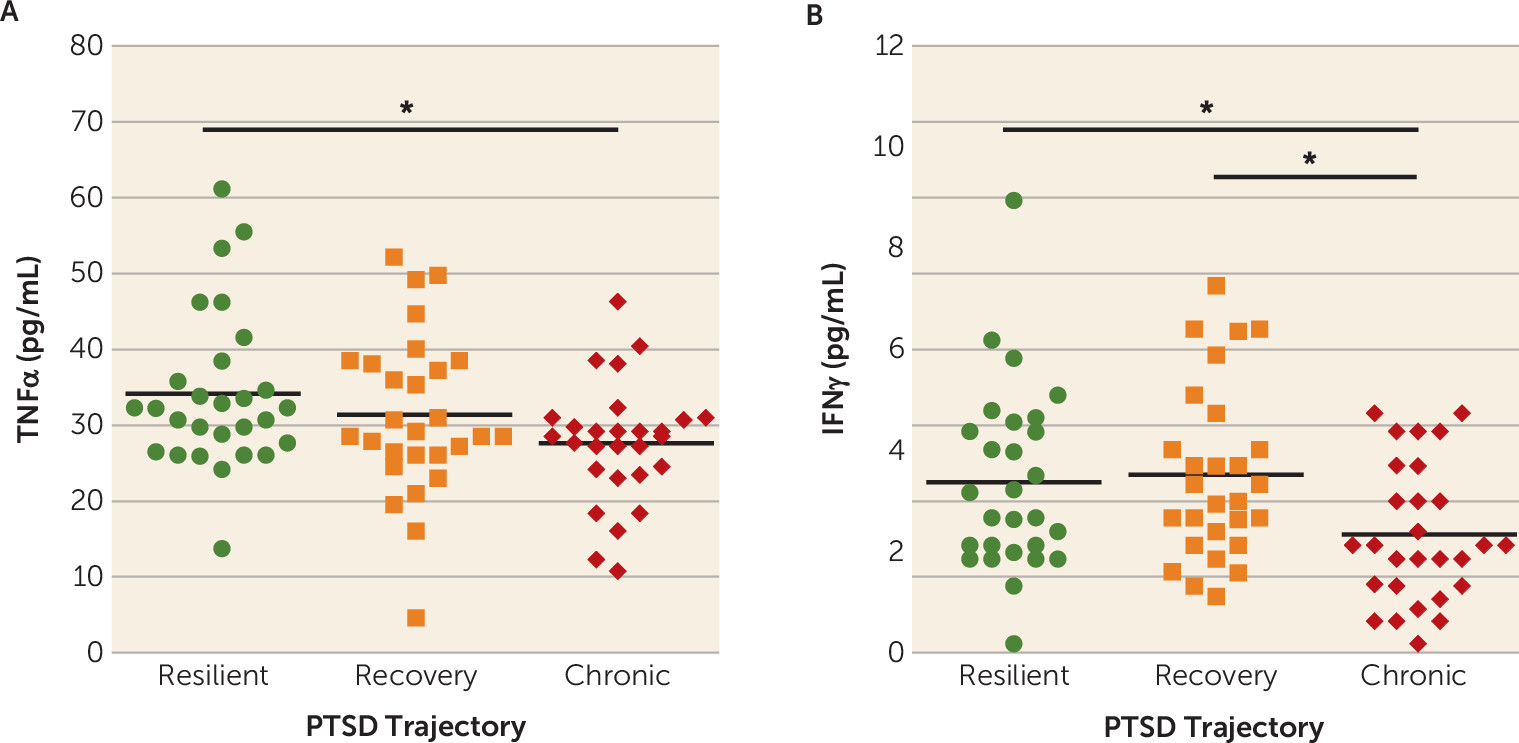
Our results indicate that lower plasma concentrations of the proinflammatory markers TNFα and IFNγ in the immediate aftermath of trauma are associated with greater risk for developing chronic PTSD symptoms. To our knowledge, this is the first prospective examination of the association between immunological activity in response to trauma exposure and subsequent risk for PTSD development. Although our results suggest that lower TNFα and IFNγ concentrations may confer individual risk for the development of chronic PTSD, further studies are necessary to characterize the potential mechanisms by which immunological changes following trauma exposure influence the development of PTSD. Overall, our results highlight the feasibility and applicability of assessing immune signals in the acute aftermath of trauma to assess prospective PTSD risk in recently traumatized individuals, while also highlighting the importance of sample collection timing for assessing prospective PTSD risk (e.g., before or immediately after trauma exposure).
Because of the practical difficulties in obtaining resting-state blood samples in large numbers of individuals before they are exposed to trauma, we designed our study specifically to assess whether inflammatory signals collected in an emergency department setting immediately after trauma exposure predict chronic PTSD development. Based on the literature linking a proinflammatory state to increased cross-sectional risk for PTSD in traumatized individuals (
8) and to increased prospective risk for PTSD development in a pre- and postdeployment study (
18), we had hypothesized that heightened inflammation in the immediate posttraumatic period would increase risk for the development of PTSD. Our data, however, showed that lower TNFα and IFNγ concentrations in the immediate aftermath of trauma were predictive of greater PTSD symptom development. These results, unexpected on the basis of the existing literature, are reminiscent of when it was shown that individuals with PTSD show enhanced, as opposed to diminished, glucocorticoid negative feedback inhibition of the hypothalamic-pituitary-adrenal (HPA) axis (
24). The most likely explanation for the discrepancy between previous findings and our results in the present study is the timing of the blood sample collection, as previous studies assessed resting-state, unprovoked concentrations of inflammatory markers in individuals with PTSD (
25–
27). However, our study design does not allow us to rule out the possibility that individuals at risk for PTSD may have lower baseline concentrations of TNF and IFNγ prior to trauma exposure.
Blood samples in this study were collected, on average, about 3 hours after exposure to a traumatic event, reflecting a state of innate immune system activation. Exposure to a stressor results in the activation of the sympathetic nervous system and the release of norepinephrine, which in turn induces nuclear factor-kappa B (NF-κB) activation and subsequent stimulation of proinflammatory cytokine production from the immune system (
28). In the context of stress-induced activation of inflammatory responses, the lower concentrations of TNFα and IFNγ in the present study may reflect a blunted activation of the immune system in the aftermath of trauma, similar to previous studies showing that blunted cortisol responses to trauma exposure confer increased risk for PTSD development in survivors of sexual assault (
29). Previous studies in humans indicate that blunted immune activation in response to stressor exposure is associated with greater cortisol reactivity to a stressor (
30) or habituation to repeated exposure to stressors (
31,
32), both of which may predispose to later PTSD responses. Similarly, findings from rodent studies show that immune deficiency following a stress exposure results in higher posttraumatic anxiety and startle responses (
33,
34). Our data also underscore the possibility that diminished glucocorticoid negative feedback inhibition of the HPA axis acutely in the aftermath of trauma may contribute to the low concentrations of TNFα and IFNγ seen in participants who went on to develop chronic PTSD in this study. Taken together, these data suggest that low inflammation may have adverse consequences on mental health outcomes, a notion that is supported by findings showing that anti-inflammatory treatment for depression is effective in individuals with preexisting high levels of inflammation, whereas such treatment can increase affective symptoms in individuals with lower baseline levels (
35).
We also assessed whether posttraumatic concentrations of anti-inflammatory cytokines, chemokines, or immune system growth factors were associated with risk for chronic PTSD symptom development. Previous studies have shown that higher concentrations of anti-inflammatory cytokines confer resilience to PTSD (
11,
13) and that higher concentrations of chemokines and immune system growth factors are associated with chronic PTSD (
10,
11). However, in this study, we did not find any associations between concentrations of anti-inflammatory factors, other cytokines, chemokines, and immune system growth factors assessed about 3 hours after trauma exposure and chronic PTSD symptom development. These null findings do not discount the possibility that increases in these cytokines, chemokines, and growth factors may occur on a different time course in the aftermath of trauma exposure and contribute to the development of PTSD. Moreover, a few studies have implicated lower chemokine production with increased stress reactivity (
36), and GM-CSF administration in survivors of acute respiratory distress syndrome has been found to result in increased PTSD symptoms (
37). Thus, further studies assessing the role of anti-inflammatory cytokines, chemokines, and immune system growth factors over time in the development of PTSD are warranted.
In summary, this study is the first prospective account linking concentrations of proinflammatory cytokines measured in the immediate aftermath of trauma exposure to increased risk for the development of chronic PTSD symptoms in civilian trauma survivors. The findings suggest that assessing immunological changes in response to trauma exposure in the emergency department may help identify patients who are most at risk for developing chronic PTSD symptoms in the aftermath of trauma. Such individuals may benefit from immediate psychological and pharmacological interventions that have been shown to be effective in attenuating PTSD development (
5,
6). It is worth considering that altered immune activity in the context of trauma exposure may also serve as a prognostic indicator of treatment response, similar to what has been described in depression (
38). While our results suggest a promising link between blunted immunological changes in the immediate aftermath of trauma exposure and chronic PTSD risk, further studies are needed to replicate and extend the generalizability of the findings.