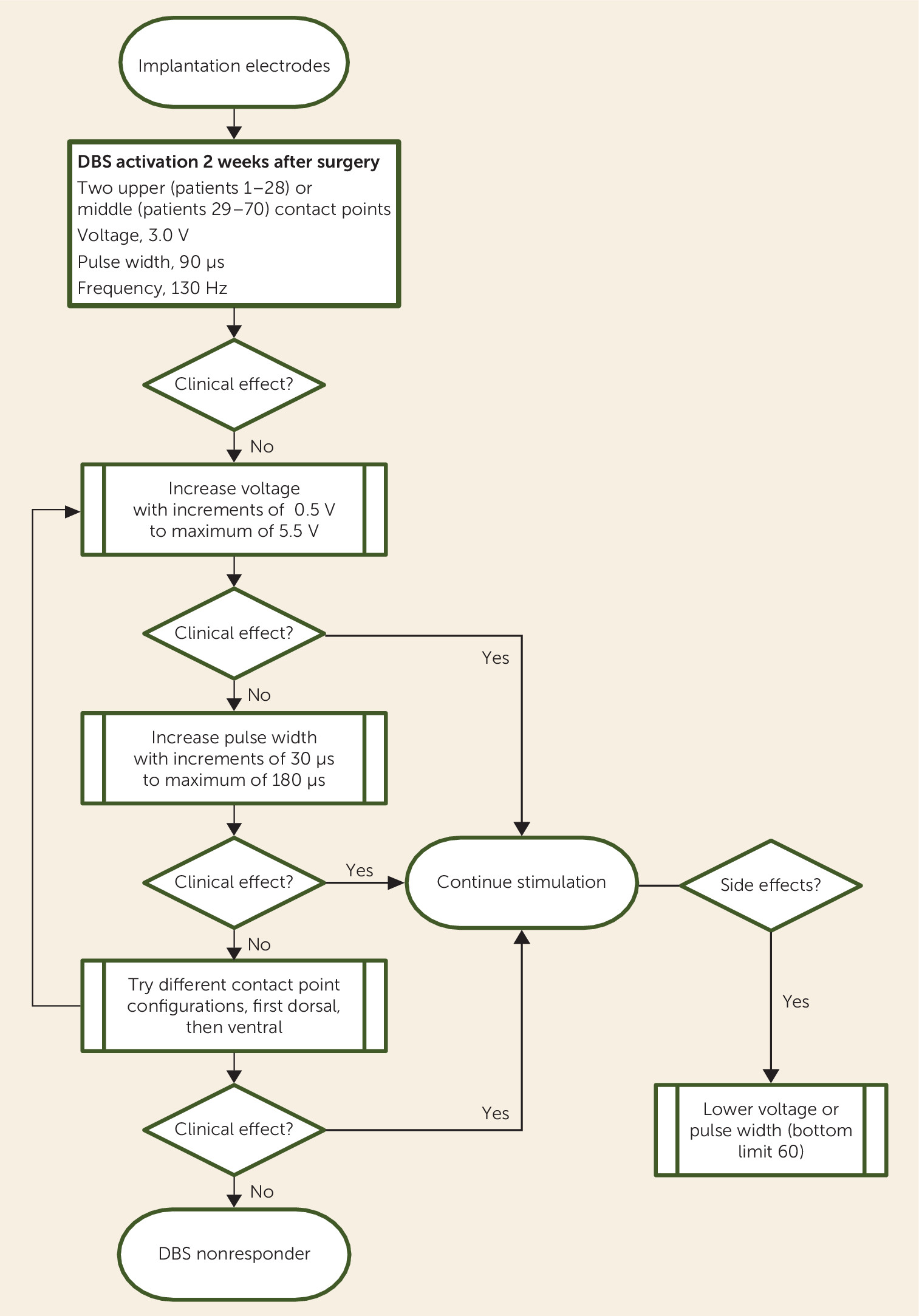
Obsessive-compulsive disorder (OCD) is characterized by persistent thoughts (obsessions) and repetitive, ritualistic behaviors (compulsions) and has an estimated lifetime prevalence of 2%. If left untreated, OCD may have a profound negative impact on a person’s capacity to function normally. Regular treatment, such as pharmacotherapy and cognitive-behavioral therapy (CBT), reduces symptoms by 40%−60% in half of patients with the disorder (
1). Even with the best available treatments, 10% of patients remain severely affected and experience treatment-refractory OCD (
1).
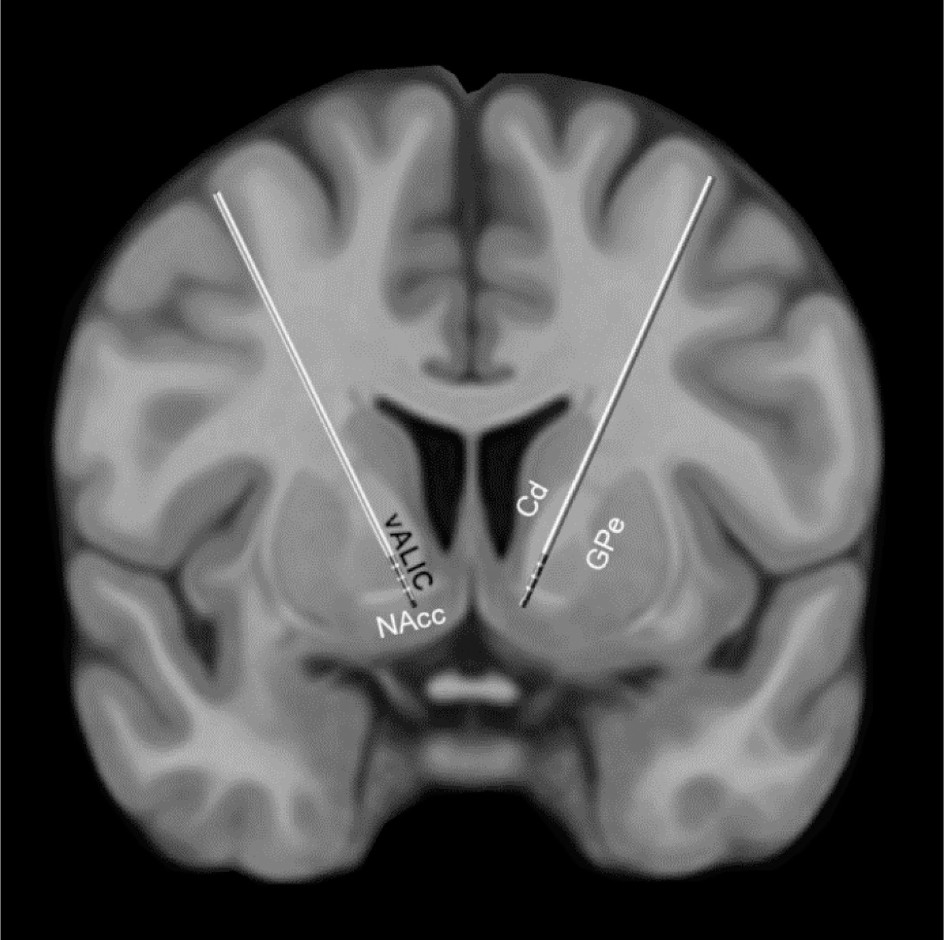
In the search for novel methods of treating refractory OCD, pioneering studies have investigated deep brain stimulation (DBS). DBS is a treatment involving implantation of electrodes, which send electrical impulses to specific brain locations with the aim of attenuating altered activity in affected circuits. Stimulation targets for DBS in patients with OCD include the ventral anterior limb of the internal capsule (vALIC), ventral striatum, subthalamic nucleus, and nucleus accumbens (NAcc) (
2–
4).
Despite the efficacy and tolerability of DBS and its potential to improve the lives of many patients with refractory OCD, evidence for the effectiveness of DBS in OCD remains limited. Few studies examining the efficacy of DBS for OCD have been published, and the total number of OCD patients treated with DBS worldwide probably does not exceed 250. In addition, the majority of studies on DBS in OCD are case or pilot studies, and the average sample size of all studies is <5 (
5). Most studies have been executed in strict experimental settings, often sponsored by medical companies. Consequently, there is an urgent need for large and company-independent clinical studies examining the effectiveness of DBS for OCD.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the 70 study participants (male, N=22; female, N=48) are presented in
Table 1. At DBS implantation, the average age of the participants was 42 years (SD=11), and the average duration of illness was 25 years (SD=11). The most common OCD subtypes were fear of contamination and cleaning (43%) and perfectionism, symmetry, and rituals (27%). The average baseline scores on the Y-BOCS and HAM-D were 34 (SD=3) and 21 (SD=6), respectively. The most prevalent comorbid disorders included major depressive disorder (43%) and obsessive-compulsive personality disorder (10%). At baseline, 86% of patients were receiving pharmacotherapy.
Clinical Outcome
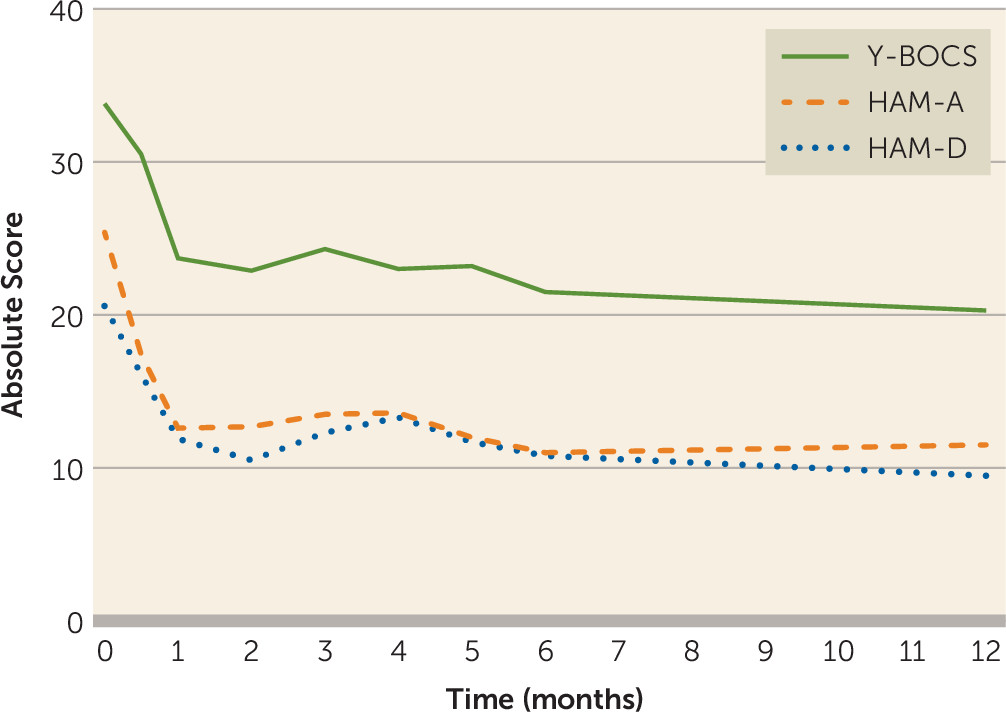
The 12 months of DBS resulted in a mean decrease in Y-BOCS scores of 13.5 points (SD=9.4) (40% reduction; Cohen’s d=1.5). We found a significant effect of stimulation on Y-BOCS scores (β=−9.54, 95% CI=−11.98, −7.11, p<0.001) and time on Y-BOCS scores (β=−0.34, 95% CI=−0.57, −0.11, p=0.004), showing that OCD symptoms decreased after active stimulation and further decreased over time.
Categorical analysis revealed that after 12 months of DBS, 36 patients were categorized as responders (52%), with a mean Y-BOCS decrease of 20.9 points (SD=6.4) (62%). Twelve patients were partial responders (17%), with a mean Y-BOCS decrease of 9.9 points (SD=1.5) (29%), and 22 patients were nonresponders (31%), with a mean Y-BOCS decrease of 3.3 points (SD=3.0) (10%).
Between baseline and the 12-month follow-up, the mean HAM-A score decreased by 13.4 points (SD=9.7) (55%; Cohen’s d=1.4), and the mean HAM-D score decreased by 11.2 points (SD=8.8) (54%; Cohen’s d=1.3). We found a significant effect of stimulation on HAM-A scores (β=−12.94, 95% CI=−15.27, −10.61, p<0.001) and on HAM-D scores (β
=−9.23, 95% CI=−11.13, −7.33, p<0.001). However, we found no significant effect of time on HAM-A and HAM-D scores, showing that stimulation had an immediate effect on affective symptoms. The course of Y-BOCS, HAM-A, and HAM-D scores during the first year of DBS is presented in
Figure 3.
Tolerability and Adverse Events
The DBS system was explanted in two patients as a result of postsurgical infection of the electrodes (implantable pulse generator, N=1; implantable pulse generator and extension cables, N=1). Both patients underwent reimplantation of the electrodes or implantable pulse generator and extension cables 3 months later. Six patients had to undergo reimplantation or retraction of the electrodes within 8 months after the initial surgery, because a postoperative CT scan fused with the preoperative MRI revealed that the electrodes were not well rooted in the vALIC (bilaterally, N=3; unilaterally, N=1) or were implanted too deeply into the NAcc (bilaterally, N=1; unilaterally, N=1) or that there was significant upward migration along the trajectory after accurate initial implantation (N=1). Two patients were treated with oral antibiotics as a result of superficial infection of one of the cranial incisions.
The main stimulation-related adverse events were transient hypomanic symptoms (39%), including restlessness (33%), agitation (30%; 3% permanent), impulsivity (19%), and sleeping disorders (46%; 7% permanent) (see Table S1 in the online supplement). Most of these symptoms were related to changes in stimulation and lasted between several days and several weeks. Other adverse events were headache (36%), pain around the burr holes (17%), feeling the implantable pulse generator in the chest (16%), pulling of the extension leads (30%), and paresthesia (20%) (e.g., at the scalp). We observed that temporary disruptions of the stimulation led to severe anxiety and depression.
Three of the 70 patients in the study sample attempted suicide, all by overdose. One attempt was classified as a serious stimulation-related adverse event because the attempt occurred 1 day after a DBS voltage increase of 0.5 V. The patient reported that she attempted suicide because she was disappointed in her symptom response to DBS. The patient’s suicidal ideation resolved without changes to the stimulation settings. The other two suicide attempts were related to comorbid bipolar disorder and borderline personality disorder. These attempts occurred at least 3 months after the last change in stimulation settings. All of the suicide attempts were without any lasting adverse consequences and did not reoccur during follow-up.
Discussion
To the best of our knowledge, our study comprising 70 patients is the largest cohort study to examine the outcome of patients receiving DBS for refractory OCD. We demonstrated that DBS of the vALIC is effective for treatment-refractory OCD and well tolerated overall, confirming the results of our previous study (
4).
In 12 months, obsessive-compulsive symptoms decreased on average by 40%, and more than half of patients were categorized as responders. Both anxiety and depression symptoms decreased rapidly by more than 50%. These results are comparable to the outcomes of our initial randomized controlled trial, in which 56% of patients were DBS responders and OCD symptoms decreased on average by 52% over the 21-month treatment period (
4). In their meta-analysis, Alonso et al. (
12) reported an average Y-BOCS score decrease of 45% (95% CI=29, 61; N=66 from 13 studies) and a responder ratio of 60% (95% CI=49, 69; N=105 from 16 studies). The symptom decrease and responder ratio in the present study were slightly lower than the estimated meta-analytic average but were well within the range reported in Alonso and colleagues’ meta-analysis. Outcomes of DBS when integrated in clinical care thus seem comparable to outcomes in controlled experimental studies, which indicates the applicability of DBS in regular clinical care. The small numerical differences may be explained by sample bias in earlier controlled experimental studies, given that our inclusion criteria were more wide-ranging; patients with comorbidities, such as bipolar disorder, autism, and severe personality disorders, were included in our study. However, to our knowledge, it has not yet been examined whether comorbidities, or other baseline patient and illness characteristics, are predictors of clinical outcome after DBS. Results from this study are also comparable to outcomes after surgical interventions, such as anterior cingulotomy (
13) and anterior capsulotomy (
14), in OCD patients.
Replicating our previous study, we found that DBS of the vALIC had a strong and immediate anxiolytic and antidepressant effect. Affective symptoms decreased rapidly after DBS and remained stable over time. Anxiolytic and antidepressant effects were observed in all responders and even in some nonresponders. This may be the reason why no patient requested that stimulation be discontinued during the follow-up, despite lack of response with obsessive-compulsive symptoms in some cases. Previous studies targeting the vALIC and ventral capsule/ventral striatum found similar effects of DBS on affective symptoms (
4,
15,
16). This effect seems to be restricted to DBS of the internal capsule, because stimulation of the subthalamic nucleus does not produce an antidepressant effect (
17). For patients with OCD and comorbid major depression, we argue that capsular DBS is recommended.
The overall treatment was generally well tolerated. There were eight surgery-related serious adverse events requiring a second surgery, including malpositioned electrodes (N=6) and postsurgical infection (N=2). However, none of these events induced lasting adverse consequences. For the majority of patients, the surgical procedure was without complications. Three suicide attempts were reported; of these, two were likely linked with comorbid bipolar disorder or borderline personality disorder. One attempt may have been related to DBS, although suicidal ideations resolved spontaneously in this patient without adjustment of stimulation settings. Nevertheless, it appears to be important to monitor for suicidality during DBS optimization, and research investigating suicidality in patients with vALIC DBS is needed. Overall, adverse events were comparable to what was reported in our previous study and in other trials (
4,
12). Hypomanic symptoms, however, were more prevalent in this study than in the meta-analysis by Alonso et al. (
12), but the prevalence rate was similar to that reported by Widge et al. (
18), with hypomanic symptoms observed in 45% of patients. Patients with hypomanic symptoms did not require hospitalization, and these symptoms were without lasting consequences. The prevalence of hypomanic symptoms may be explained either by our specific target or by the lack of specificity in other studies. Because DBS of the vALIC also has a strong antidepressant effect, it seems probable that hypomanic symptoms are not solely an adverse effect of DBS but are part of what makes DBS effective for OCD. However, hypomanic symptoms occurred approximately equally in both responders and nonresponders (42% of responders and 35% of nonresponders).
Our findings should be interpreted in the context of the strengths and limitations of the study. Our study population reflects the population referred to a tertiary referral center, and over time we had more wide-ranging inclusion criteria than we did previously, with the inclusion of patients with more severe comorbidities. This resulted in a sample resembling patients in routine clinical practice in a tertiary referral center. In addition, the study was completely independent and not sponsored by a medical company. To minimize selection bias, we included all of the consecutive patients who received DBS for OCD at our center. To minimize measurement bias, we reviewed the data from our prospectively collected database, and the symptom scales were administered by clinicians trained on use of the scales. Because the study was an open study, absence of a sham control group and lack of blinding may have resulted in systematic bias in our findings. A recent individual patient-data meta-analysis by Schruers et al. (
19) showed that sham stimulation induces a significant change in Y-BOCS scores. Nevertheless, the sham score reported by Schruers et al. (
19) is considerably smaller than the effect observed in our study (4.52 points compared with 13.5 points on the Y-BOCS). In addition, Schruers et al. acknowledged that sham effects are not likely to be sustained long-term, and in a subsample of the patients in the present study, we previously included a double-blind phase and still observed significant and large effects compared with a sham control condition (
4).
Transient recurrence of obsessive-compulsive symptoms was observed in patients whose batteries reached end of life. However, these transient deteriorations were not captured by our study design, which included only follow-up data at fixed time points until 1 year after implantation. Future sham-controlled trials should provide further evidence that the effects of DBS for OCD extend beyond placebo effect. Another limitation is that we included 16 patients who participated in a clinical trial, to increase power, but as a result, the sample was not purely naturalistic. In addition, we did not report the long-term (>1 year) beneficial and adverse effects of DBS for OCD. Moreover, selection regarding baseline demographic and symptom typology could improve the effectiveness of DBS. Given the extensiveness of these data, we will present the relationship between baseline factors and outcomes in a separate study. Additionally, at our treatment center, it is not standard procedure to awaken the patients during implantation surgery to evaluate behavioral effects. Haq et al. (
20) demonstrated that the presence of stimulation-induced laughter was correlated with greater improvement in OCD symptoms after 2 years of DBS. Given that our patients were not awakened during surgery, another limitation of our study is that we were not able to examine the relationship of laughter and other intraoperative mood changes and behavior with response to DBS. Finally, we did not use tractography to refine electrode placement. Our research group previously performed tractography analysis of the anterior thalamic radiation and the supero-lateral branch of the medial forebrain bundle, using diffusion-weighted MRI data for 12 patients from the present cohort (21). Active stimulation of the vALIC closer to the medial forebrain bundle than the anterior thalamic radiation was associated with better treatment outcome as measured by the Y-BOCS. Future controlled intervention studies could examine whether DBS of the medial forebrain bundle using tractography produces better symptom reduction than DBS of the vALIC.
Our study confirms our previous finding that DBS of the vALIC is an effective and tolerable treatment for refractory OCD. We emphasized the importance of adequate selection of patients, as well as modulation over an extensive period to optimize DBS settings and the use of a consolidation phase in which we applied psychoeducation and psychotherapy so that patients could cope with avoidance behaviors. These are separate treatment phases of DBS that require specialized skills and attention. We argue that none of these phases are redundant, and it may be that precisely this integrated multidisciplinary method largely contributed to the success rate in our DBS-treated cohort. Future studies could focus on specific elements of this approach to examine how each phase affects DBS outcome. Investigating predictors of success could improve DBS selection, and examining the effects of CBT may distinguish between the effects of modulation and consolidation.