20-Year Trends in the Pharmacologic Treatment of Bipolar Disorder by Psychiatrists in Outpatient Care Settings
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Data Source
Survey Methods
Medication Classification
Demographic and Clinical Data
Analysis
Results
Sample Characteristics
| Secular Trends | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure or Variable | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | Unadjusted Odds Ratio | 95% CI | p | Adjusted Odds Ratiob | 95% CI | p |
| N | N | N | N | N | |||||||
| Unweighted visits | 504 | 889 | 864 | 1,313 | 849 | ||||||
| Weighted visits (row %) | 467,057 (11.1%) | 753,228 (17.8%) | 867,632 (20.6%) | 1,074,923 (25.5%) | 1,059,311 (25.1%) | ||||||
| Mean | Mean | Mean | Mean | Mean | |||||||
| Age | |||||||||||
| ≤18 | 12.3 | 16.2 | 9.9 | 7.0 | 8.4 | 0.45 | 0.20, 0.99 | 0.047 | 0.41 | 0.20, 0.83 | 0.013 |
| 19–44 | 44.8 | 42.7 | 43.3 | 44.2 | 43.3 | 0.99 | 0.75, 1.31 | 0.947 | 1.02 | 0.77, 1.36 | 0.869 |
| 45–64 | 35.6 | 34.5 | 39.6 | 41.5 | 35.5 | 1.09 | 0.79, 1.49 | 0.597 | 1.07 | 0.78, 1.47 | 0.657 |
| ≥65 | 7.3 | 6.5 | 7.2 | 7.3 | 12.9 | 2.14 | 1.26, 3.64 | 0.005 | 2.21 | 1.28, 3.80 | 0.004 |
| Gender | |||||||||||
| Male | 40.6 | 39.1 | 32.2 | 39.6 | 36.2 | 0.91 | 0.67, 1.23 | 0.530 | 1.09 | 0.82, 1.46 | 0.562 |
| Female | 59.4 | 60.9 | 67.8 | 60.4 | 63.8 | 1.12 | 0.81, 1.50 | 0.530 | 0.92 | 0.69, 1.23 | 0.562 |
| Race/ethnicity | |||||||||||
| Non-Hispanic white | 91.4 | 87.2 | 85.5 | 51.5 | 81.3 | 0.46 | 0.29, 0.72 | 0.001 | 0.44 | 0.27, 0.71 | 0.001 |
| Non-Hispanic black | 3.6 | 5.3 | 6.0 | 7.8 | 6.1 | 1.60 | 0.95, 2.67 | 0.076 | 1.62 | 0.97, 2.73 | 0.068 |
| Hispanic | 2.6 | 4.2 | 5.9 | 7.7 | 6.2 | 2.10 | 1.18, 3.75 | 0.012 | 2.19 | 1.17, 4.09 | 0.014 |
| Non-Hispanic other | 2.5 | 3.4 | 2.5 | 3.0 | 6.4 | 2.74 | 0.99, 7.56 | 0.052 | 2.86 | 0.98, 8.29 | 0.054 |
| Insurance coverage | |||||||||||
| Private | 45.2 | 48.4 | 48.1 | 43.8 | 46.0 | 0.96 | 0.64, 1.43 | 0.833 | 1.02 | 0.68, 1.54 | 0.913 |
| Medicare | 16.1 | 12.3 | 20.0 | 17.7 | 18.6 | 1.40 | 0.96, 2.04 | 0.082 | 1.10 | 0.74, 1.62 | 0.635 |
| Medicaid | 9.1 | 17.9 | 15.5 | 17.5 | 16.4 | 1.37 | 0.75, 2.53 | 0.308 | 1.58 | 0.86, 2.90 | 0.142 |
| Other | 29.7 | 21.4 | 16.5 | 21.0 | 19.1 | 0.71 | 0.42, 1.18 | 0.180 | 0.73 | 0.44, 1.22 | 0.232 |
| Major reason for visit | |||||||||||
| Acute problem | 12.8 | 3.6 | 4.2 | 4.3 | 4.1 | 0.37 | 0.16, 0.88 | 0.024 | 0.44 | 0.19, 1.00 | 0.049 |
| Chronic problem | 85.7 | 96.2 | 94.0 | 93.7 | 94.2 | 1.38 | 0.67, 2.85 | 0.381 | 1.82 | 0.68, 2.55 | 0.411 |
| Other | 1.6 | 0.2 | 1.8 | 2.0 | 1.8 | 2.07 | 0.30, 14.45 | 0.464 | 1.70 | 0.25, 11.39 | 0.584 |
| Region | |||||||||||
| Northeast | 27.5 | 19.0 | 25.9 | 21.6 | 30.0 | 1.31 | 0.64, 2.69 | 0.455 | 1.33 | 0.67, 2.63 | 0.416 |
| Midwest | 22.5 | 21.6 | 15.4 | 21.4 | 19.3 | 0.9 | 0.46, 17.76 | 0.752 | 0.98 | 0.49, 1.96 | 0.959 |
| South | 27.6 | 36.0 | 38.6 | 35.0 | 30.6 | 0.96 | 0.53, 1.76 | 0.903 | 0.95 | 0.51, 1.76 | 0.862 |
| West | 22.4 | 23.4 | 20.2 | 22.0 | 20.1 | 0.86 | 0.45, 1.65 | 0.656 | 0.8 | 0.42, 1.51 | 0.487 |
| Time spent with doctor | |||||||||||
| ≤15 minutes | 22.6 | 21.0 | 37.6 | 24.8 | 25.1 | 1.09 | 0.63, 1.87 | 0.766 | 1.09 | 0.62, 1.93 | 0.756 |
| 16–30 minutes | 33.0 | 45.1 | 35.5 | 44.2 | 45.6 | 1.45 | 0.93, 2.26 | 0.101 | 1.47 | 0.94, 2.30 | 0.094 |
| >30 minutes | 44.5 | 33.9 | 26.9 | 31.0 | 29.3 | 0.62 | 0.40, 0.96 | 0.033 | 0.59 | 0.36, 0.98 | 0.039 |
| Comorbid disorders | |||||||||||
| Anxiety disorder | 11.5 | 17.3 | 18.9 | 16.0 | 24.3 | 1.88 | 1.13, 3.14 | 0.015 | 1.85 | 1.10, 3.11 | 0.020 |
| Psychotic disorder | 0.9 | 1.5 | 1.6 | 2.4 | 2.2 | 2.14 | 0.56, 8.15 | 0.265 | 1.91 | 0.52, 7.08 | 0.331 |
| Substance use disorder | 7.3 | 7.1 | 7.8 | 10.7 | 9.8 | 1.57 | 1.01, 2.46 | 0.047 | 1.53 | 0.95, 2.46 | 0.078 |
| Other | 4.3 | 5.0 | 4.1 | 4.2 | 4.5 | 0.95 | 0.48, 1.88 | 0.885 | 0.91 | 0.45, 1.83 | 0.783 |
| % | % | % | % | % | |||||||
| Metropolitan Statistical Area | 83.8 | 89.5 | 90.7 | 93.3 | 95.4 | 3.61 | 1.38, 9.45 | 0.009 | 3.96 | 1.42, 11.07 | 0.009 |
| Psychotherapy | 50.9 | 57.3 | 46.8 | 50.8 | 35.7 | 0.50 | 0.30, 0.82 | 0.007 | 0.44 | 0.26, 0.73 | 0.001 |
Prescriptions for Psychotropic Medications
Antipsychotics.
| Secular Trends | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure or Variable | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | Unadjusted Odds Ratio | 95% CI | p | Adjusted Odds Ratiob | 95% CI | p |
| N | N | N | N | N | |||||||
| Unweighted visits | 504 | 889 | 864 | 1,313 | 849 | ||||||
| Weighted visits (row %) | 467,057 (11.1%) | 753,228 (17.8%) | 867,632 (20.6%) | 1,074,923 (25.5%) | 1,059,311 (25.1%) | ||||||
| Mean | Mean | Mean | Mean | Mean | |||||||
| Any antipsychotic | 19.1 | 31.8 | 51.5 | 53.0 | 52.7 | 3.80 | 2.77, 5.21 | <0.001 | 4.00 | 2.94, 5.45 | <0.001 |
| First-generation antipsychotics | 7.0 | 3.1 | 2.8 | 3.1 | 1.7 | 0.32 | 0.17, 0.60 | <0.001 | 0.23 | 0.12, 0.45 | <0.001 |
| SGAs | 12.4 | 29.4 | 49.6 | 51.4 | 51.4 | 4.64 | 3.34, 6.46 | <0.001 | 5.05 | 3.65, 7.01 | <0.001 |
| FDA-approved SGAs | 12.1 | 28.2 | 49.1 | 48.6 | 50.1 | 4.43 | 3.18, 6.19 | <0.001 | 4.80 | 3.45, 6.69 | <0.001 |
| Any mood stabilizer | 62.3 | 50.3 | 28.5 | 23.7 | 26.4 | 0.20 | 0.14, 0.29 | <0.001 | 0.18 | 0.13, 0.27 | <0.001 |
| Lithium | 30.4 | 20.7 | 17.3 | 13.9 | 17.6 | 0.50 | 0.33, 0.76 | 0.001 | 0.46 | 0.29, 0.71 | <0.001 |
| Carbamazepine or valproic acid | 35.4 | 24.8 | 7.6 | 7.4 | 4.9 | 0.07 | 0.05, 0.11 | <0.001 | 0.07 | 0.04, 0.11 | <0.001 |
| Lamotrigine | 2.1 | 9.7 | 5.8 | 4.1 | 4.7 | 0.73 | 0.46, 1.15 | 0.173 | 0.70 | 0.44, 1.12 | 0.137 |
| Other anticonvulsants | 6.3 | 19.9 | 12.3 | 12.3 | 12.5 | 0.96 | 0.62, 1.48 | 0.859 | 0.91 | 0.59, 1.41 | 0.684 |
| Gabapentin | 6.0 | 9.1 | 3.9 | 4.1 | 4.7 | 0.53 | 0.34, 0.85 | 0.008 | 0.45 | 0.28, 0.73 | 0.001 |
| Oxcarbazepine | 0.0 | 5.7 | 4.5 | 3.3 | 5.3 | 1.85 | 0.83, 4.13 | 0.134 | 1.82 | 0.82, 4.03 | 0.143 |
| Topiramate | 0.3 | 5.7 | 4.5 | 5.0 | 3.1 | 1.20 | 0.75, 1.93 | 0.449 | 1.28 | 0.76, 2.15 | 0.345 |
| Any antidepressant | 47.0 | 50.2 | 55.1 | 49.9 | 57.5 | 1.37 | 1.02, 1.84 | 0.036 | 1.27 | 0.93, 1.75 | 0.133 |
| TCAs or MAOIs | 6.2 | 3.5 | 3.1 | 2.2 | 2.9 | 0.44 | 0.20, 0.98 | 0.044 | 0.36 | 0.17, 0.77 | 0.009 |
| SSRIs | 24.0 | 31.4 | 30.7 | 26.0 | 25.7 | 0.87 | 0.65, 1.16 | 0.349 | 0.85 | 0.63, 1.14 | 0.272 |
| SNRIs | 4.5 | 6.6 | 13.7 | 13.0 | 11.4 | 2.16 | 1.41, 3.31 | <0.001 | 1.98 | 1.33, 2.96 | 0.001 |
| Other | 19.0 | 17.5 | 19.8 | 19.2 | 29.8 | 1.94 | 1.33, 2.84 | 0.001 | 1.86 | 1.22, 2.84 | 0.004 |
| Bupropion | 9.8 | 11.8 | 10.9 | 10.4 | 12.4 | 1.15 | 0.78, 1.69 | 0.488 | 1.13 | 0.75, 1.72 | 0.560 |
| Mirtazapine | 2.4 | 2.1 | 1.9 | 1.7 | 4.1 | 1.96 | 0.90, 4.28 | 0.092 | 1.69 | 0.76, 3.78 | 0.201 |
| Other | 8.4 | 5.0 | 7.8 | 8.4 | 15.8 | 3.06 | 1.76, 5.32 | <0.001 | 2.93 | 1.62, 5.30 | <0.001 |
| Antidepressant without mood stabilizer | 17.9 | 20.7 | 38.9 | 35.6 | 40.9 | 2.98 | 2.16, 4.11 | <0.001 | 2.88 | 2.06, 4.03 | <0.001 |
| Antidepressant without antipsychotic | 38.0 | 34.8 | 28.7 | 21.9 | 25.5 | 0.49 | 0.36, 0.67 | <0.001 | 0.45 | 0.33, 0.61 | <0.001 |
| Antidepressant without mood stabilizer or antipsychotic | 14.9 | 13.0 | 19.5 | 14.2 | 16.6 | 1.13 | 0.78, 1.63 | 0.512 | 1.05 | 0.73, 1.51 | 0.793 |
| Benzodiazepines | 24.2 | 27.6 | 30.3 | 33.4 | 31.2 | 1.41 | 1.00, 1.97 | 0.047 | 1.30 | 0.95, 1.78 | 0.097 |
| Stimulants | 5.3 | 3.5 | 6.3 | 7.8 | 9.8 | 2.67 | 1.38, 5.16 | 0.004 | 2.75 | 1.44, 5.27 | 0.002 |
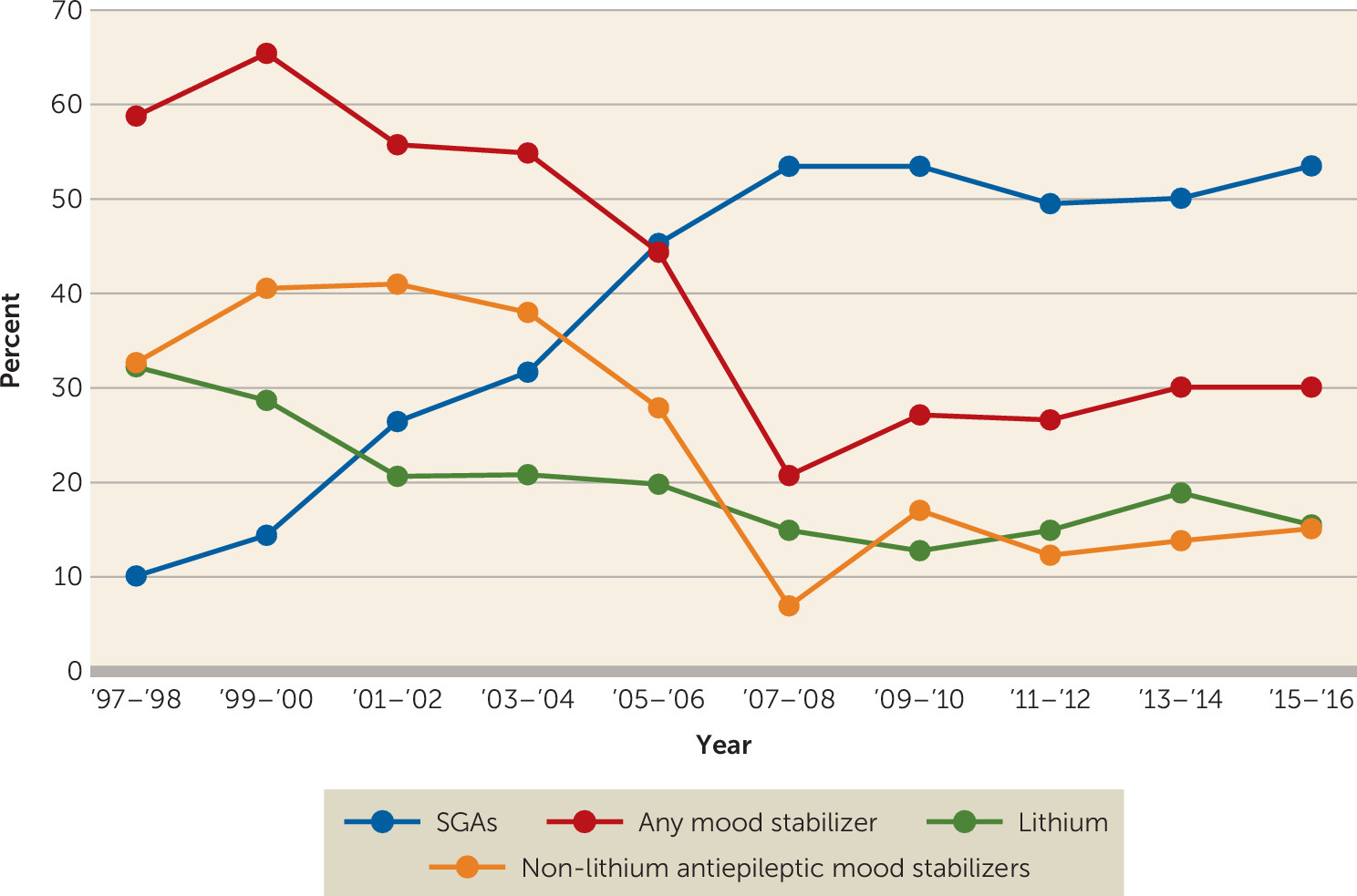
Mood stabilizers.
Antidepressants.
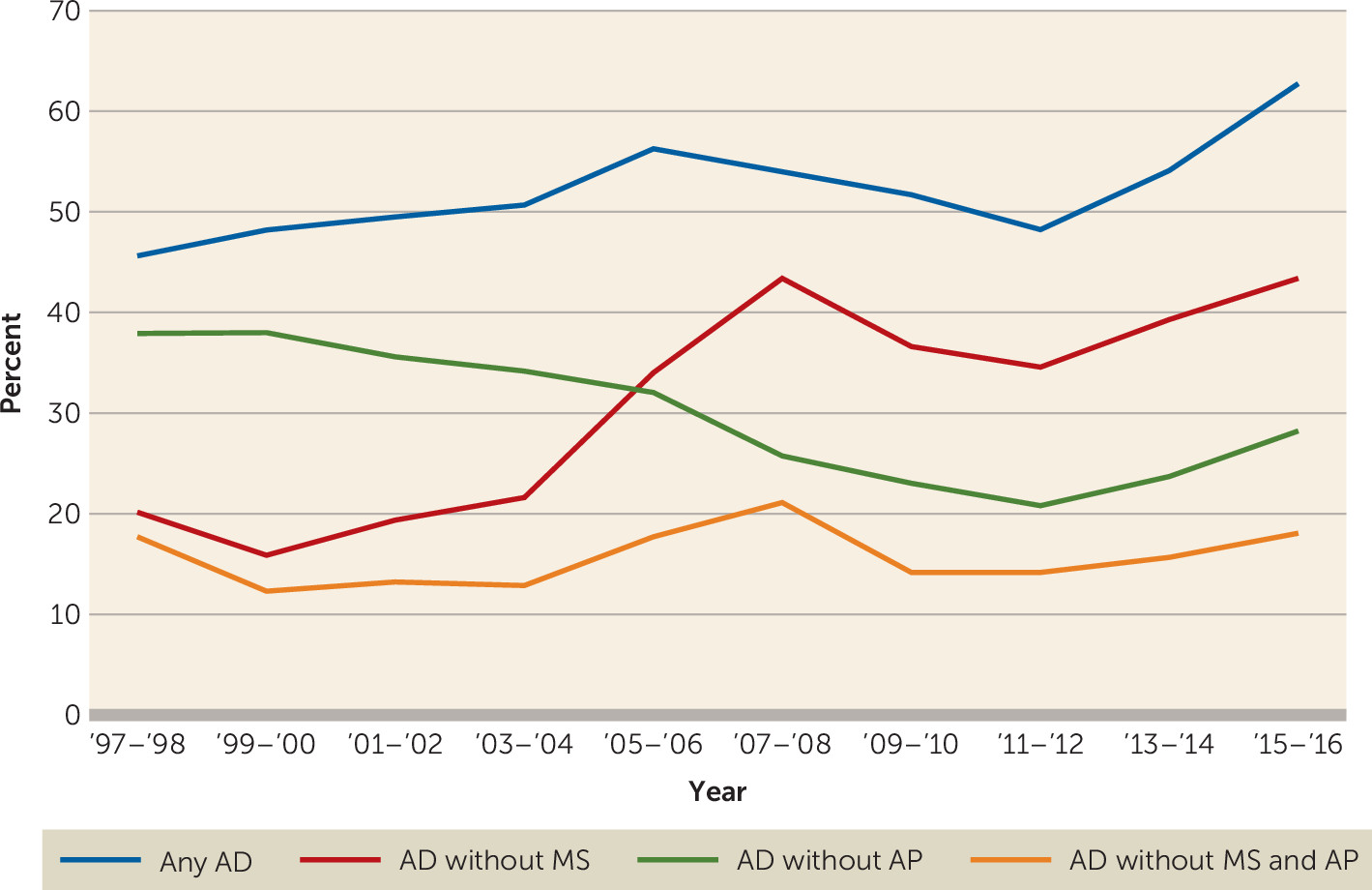
Other psychotropic medication use.
Unopposed antidepressant use.
Psychotherapy
Discussion
Second-Generation Antipsychotics and Mood Stabilizers
Implications for Public Health
Antidepressant Use in Bipolar Disorder
Use of Psychotherapy
Limitations
Conclusions
Supplementary Material
- View/Download
- 452.22 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

