According to the 2017 National Survey on Drug Use and Health (
1), the prevalence of mental illness is higher among women (22.3%) than men (15.1%), and young adults 18–25 years of age have the highest prevalence. These statistics highlight the frequency of psychiatric disorders during the reproductive years in women. A critical element of mental health care is decision support in anticipation of major life events such as pregnancy and parenting. Psychiatrists are well positioned to support women by encouraging contraception until pregnancy is desired, providing education on the reproductive health effects of psychotropic medications, and discussing the course of mental illness across childbearing. Women who access mental health services need sexual and reproductive health information (
2).
Contraception is an essential component of preventive health care. In the United States, 45% of pregnancies are unplanned. Large disparities in the rate of unintended pregnancy exist, with higher rates among women who are unmarried, have poor social support, are members of racial/ethnic minority groups, and have mental illness (
3). Unintended pregnancy is associated with new episodes of psychiatric disorders, including perinatal depression (
4). Once an episode occurs, a recurrent cycle of risk evolves. Women with current or past depressive symptoms have an elevated likelihood of unintended pregnancies, with more than a fivefold increase in risk because of a greater probability of using contraceptive methods of low as opposed to moderate effectiveness, independent of race/ethnicity, age, partner status, and parity (
5). Anhedonia, cognitive impairment, limited capacity for risk assessment, and poor understanding of sexual physiology may make consistent and correct contraceptive use unmanageable (
4).
Unintended pregnancies pose significant health risks for women with poorly controlled mental illness. Pregnancy, birth, and infant care force adjustments in interpersonal, occupational, and financial functioning that challenge coping skills and resources. Effective pharmacotherapy may be discontinued proximate to conception, which increases the risk for recurrence of psychiatric symptoms. Even when pharmacotherapy is maintained, the dynamic physiology of pregnancy may alter plasma drug concentrations and compromise efficacy. The postpartum period is associated with an elevated risk of emergence of mood disorders in women sensitive to rapid hormonal change. Psychiatric illness instability increases health risks for the maternal-fetal dyad, the newborn, and the family.
Our goal in this study, as a team of psychiatrists and obstetrician-gynecologists, is to provide clinicians with information to improve collaboration in the contraceptive care of women with psychiatric disorders. Managing mental illness to sustain a woman’s capacity to function optimally, adapt to the challenges of pregnancy, and prepare for birth is critical to her (and her family’s) well-being. Modern methods of birth control allow women with psychiatric disorders to delay pregnancy until their capacity to manage their mental and reproductive health is optimized. We provide an overview of contraceptive choices, a summary of the impact of hormonal contraceptives on mood symptoms and psychotropic drugs, and guidance for the application of this information to women with psychiatric disorders.
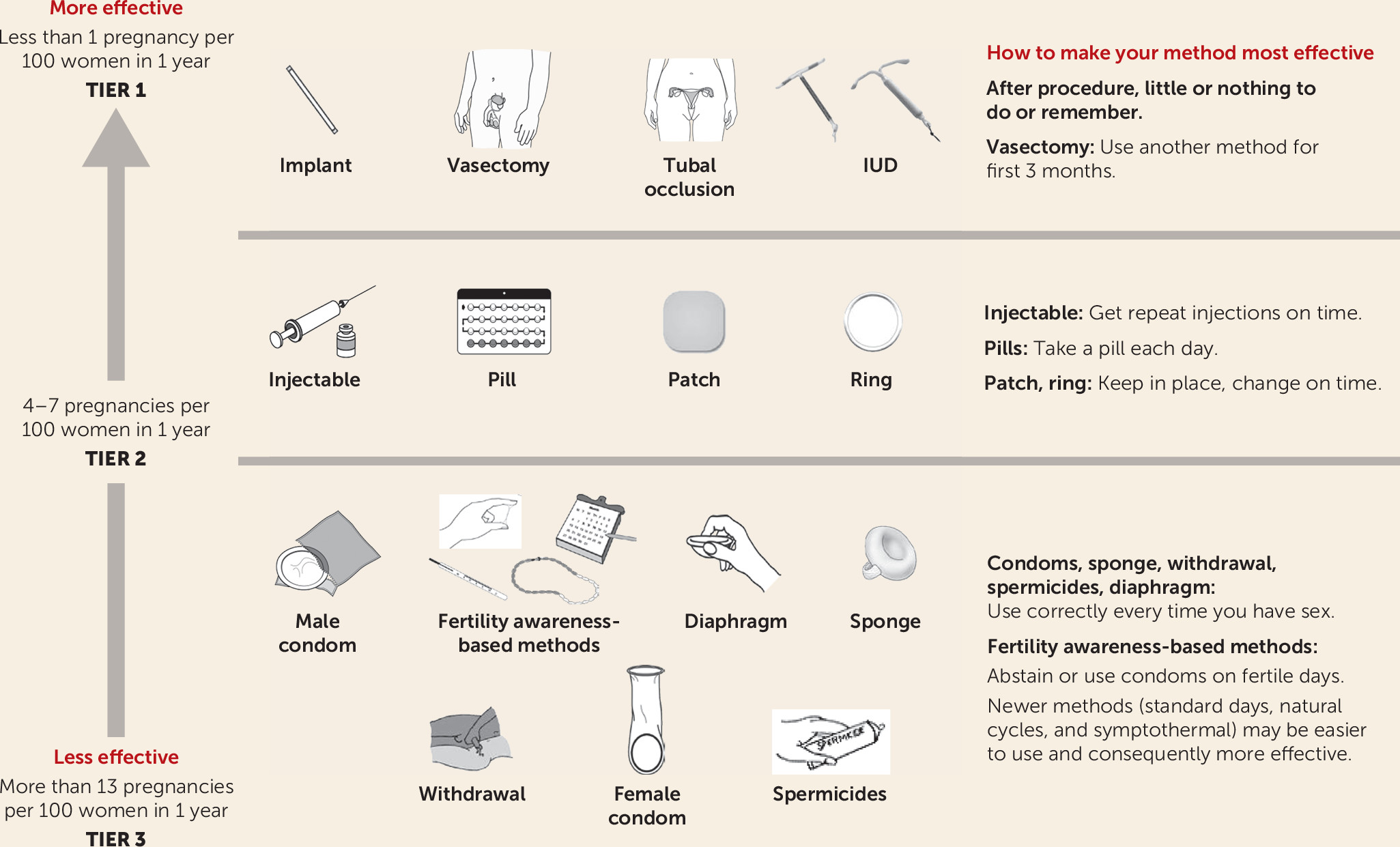
The U.S. Medical Eligibility Criteria for Contraceptive Use (
6), developed by the Centers for Disease Control and Prevention, is an educational tool and comprehensive guide. The Medical Eligibility Criteria were used as the framework for our review of clinical decision making in contraceptive care. The various contraceptive methods, grouped by effectiveness, are shown in
Figure 1. The Medical Eligibility Criteria also describe the suitability of each method for specific medical disorders (with a convenient summary chart) that are common comorbidities with psychiatric conditions. Depression is included with the guidance that all contraceptive methods are appropriate, although comorbidities may dictate the method.
Methods
We performed a literature search on contraceptives for women with psychiatric disorders in PsycINFO, PubMed, Embase, and Scopus. We included the following search terms coupled with the term contraceptive: psychiatry, depression, anxiety, mood, schizophrenia, bipolar disorder, psychotropic drugs, anticonvulsants, antidepressants. We also searched for psychiatric disorder with the terms contraception, depot medroxyprogesterone acetate, progestin, and intrauterine device. Publications were selected by the authors’ consensus that the article contributed data supporting evidence-based care relevant for psychiatrists who treat women with contraceptive needs. All authors participated in developing the consensus document by iterative draft review and integration of the interdisciplinary literature.
Results
Overview of Contraceptive Choices
Long-Acting Reversible Contraceptives (LARCs).
LARCs include subdermal implants and intrauterine devices (IUDs). LARCs are associated with low failure rates, favorable safety profiles, few contraindications, cost-effectiveness, and rapid return to fertility after removal. Both the American College of Obstetricians and Gynecologists (ACOG) (
7) and the American Academy of Pediatrics (
8) encourage access to LARCs, which are appropriate for the majority of women, including adolescents and nulliparas. The subdermal implant is a progestin-containing rod that is effective for 3 years, with a failure rate of 0.1% (
9). Most women experience amenorrhea or light menstrual bleeding, although 20% report irregular bleeding (
10). Five IUDs (one nonhormonal copper and four hormone-releasing devices) are available in the United States. They provide contraception for 3 to 10 years, with less than a 1% failure rate (
9). The hormone-releasing IUDs contain the progestin levonorgestrel, which acts locally in the uterus; however, it is measurable in plasma at concentrations much lower than produced by otherlevonorgestrel-containing contraceptives (
11). The IUD confers benefits such as reducing heavy menstrual bleeding as well as endometrial protection for women with anovulatory cycles. Despite their effectiveness, LARCs are chosen by only 14% of contraceptive-using American women (
12). The relatively low rate of IUD use stems from persistent concerns about pelvic infections associated with IUDs that are no longer available, fear of pain with placement, changes in menstrual bleeding, and perceived lack of control over IUD removal (
13). Pregnancy rates after removal of current IUDs are similar to those after discontinuation of other contraceptives (
14). Emphasis on the effectiveness of LARC methods for long-term pregnancy prevention has raised concerns about patient autonomy, trust in the health care system, and reproductive oppression (
15). Clinicians and policy makers have the responsibility to avoid assumptions about contraceptive care based on age, race, ethnicity, economic status, sexual orientation, gender identity, or disability. Provision of information about all contraceptive options and decision-making support for personal circumstances is an optimal approach. Equity in contraceptive provision is an active area of research.
Progestin-Only Contraceptives.
These include the progestin (etonogestrel) subdermal implant (also a LARC), a progestin-only oral formulation, the levonorgestrel IUD, and depot medroxyprogesterone acetate (DMPA). The traditional progestin-only pill (the “minipill”) contains norethindrone and must be taken at the same time every day for optimal efficacy. The progestin drospirenone, developed for its antiandrogenic effects, is available in pill form, and its efficacy does not depend on the timing of the daily dose. It was combined with ethinyl estradiol as a contraceptive and treatment for premenstrual dysphoric disorder, acne, and hirsutism. The typical failure rate for progestin-only pill preparations is 7% (
9). Progestin-only formulations are appropriate during lactation and for women with contraindications to estrogen-containing products, such as thromboembolism or stroke.
DMPA is administered by intramuscular injection every 12–14 weeks. DMPA has a failure rate of 4%, primarily due to inability to attend injection appointments (
16). The non-contraceptive benefits of DMPA include decreased menstrual bleeding, relief of dysmenorrhea, and prevention of ovarian cysts. Adverse events include irregular bleeding and a reversible decrease in bone mineral density. Compared to women treated with nonhormonal or oral contraceptives, DMPA users had significantly greater increases in body weight (5.1 kg) and body fat (3.4%) over a 3-year period. Weight gain associated with DMPA may be particularly problematic for cumulative weight increase in women whose psychotropic medications also are associated with this challenging side effect.
Combined Hormonal Contraceptives (CHCs).
CHCs contain an estrogen (usually synthetic ethinyl estradiol) and a progestin. They are administered orally, vaginally, or transdermally. Current preparations include combined oral contraceptives (COCs), vaginal ring, and transdermal patch. The CHCs suppress ovulation and have a failure rate of 7% (
9). CHCs offer several non-contraceptive benefits, such as regulation of the menstrual cycle, alleviation of dysmenorrhea, suppression of endometriosis, reduction of acne and hirsutism, increase in bone mass, and reduction in ovarian and endometrial cancer risk (
18). The standard regimen for CHCs parallels a 28-day menstrual cycle, with 21 days of hormone(s), followed by 7 days of an inactive pill and withdrawal bleeding.
COCs are the most commonly prescribed reversible birth control method. They are used by 21% of contraceptive users and 12.6% of all women ages 15–44 years (
19). The vaginal ring is placed for 21 days and replaced after a 7-day hiatus. The transdermal patch is applied for 3 weeks followed by a patch-free week. COC formulations are also available as extended-cycle preparations with hormone administration for longer intervals followed by fewer hormone-free weeks and bleeding periods. This type of COC results in longer periods of stable hormonal treatment and is preferable for women with somatic and mood symptoms that are sensitive to hormonal fluctuation (
20). Menstrual suppression for hygiene management may be requested by women with disabilities or by their caretakers.
Discontinuation or change in contraceptive method is frequent and in one study was reported in about 30% of women within 12 months of initiation (
21). Nearly three-quarters of users reported side effects as the reason. The most commonly reported, in order of frequency, were vaginal bleeding, mood/depression, pain/cramping, weight change, and gastrointestinal problems. A patient-centered decision making approach to contraceptive selection, with information on the risks, benefits, and potential side effects of all methods, is optimal. The contraceptive choice must be balanced with the woman’s lifestyle and medical history. A plan for short-term follow-up to assess the woman’s satisfaction with the method is appropriate given the substantial rates of discontinuation and change.
Barrier Methods.
Male condoms are widely available and provide some protection against sexually transmitted infections (
6). The failure rates for male and female condoms are 13% and 21%, respectively (
9). The diaphragm is a dome-shaped device inserted in the vagina to cover the cervix. It can be inserted up to 6 hours before coitus, remains in place for 6 hours after intercourse, and must be removed within 24 hours. The failure rate is 17% (
9).
Emergency Contraception.
Following unprotected intercourse or suspected contraceptive failure, emergency contraception decreases the risk of pregnancy by 81%−90% (
6). The most commonly used method is a single oral dose of levonorgestrel (1.5 mg) administered up to 120 hours after intercourse (
6). The U.S. Food and Drug Administration approved this method for over-the-counter availability for use by women of all ages without a prescription. A threefold increased risk of contraceptive failure occurs in women with a body mass index >30. A higher levonorgestrel dose (3 mg) may overcome this decrease in efficacy (
22). The second most commonly used method that provides the benefits of longer-term contraception is the copper IUD, which must be inserted by a clinician within 5 days of intercourse. Another option is a single dose of ulipristal acetate (30 mg) within 120 hours after intercourse. There are no significant contraindications to oral emergency contraceptive use, and no harm from repeated doses (
6). However, it is appropriately reserved for isolated episodes of inadequately protected intercourse rather than as a primary mode of contraception. It is less reliable than other methods, with a failure rate comparable to barrier methods (
6).
Permanent Sterilization.
Surgical sterilization is the most common method chosen by American women who are age 30 or older and have completed their childbearing plans (
19). Tubal ligation or bilateral salpingectomy are performed at the time of cesarean section, following vaginal delivery, or at a time unrelated to pregnancy for sterilization. The failure rate in females is 0.5%, and the vasectomy failure rate is 0.15% (
9).
Contraception and Mood Symptoms
In this section, we use the inclusive term oral contraceptives (OCs) to designate both COCs and progestin-only pill preparations.
The impact of hormonal contraceptives on the risk for the emergence of depression is a complex topic without consensus. The authors of a Danish population-based study (
23) reported that hormonal contraceptive users had an increased relative risk for initiation of antidepressant treatment. The magnitude of the association was about twofold greater for adolescents (15–19 years), and higher with progestin-only pill preparations (relative risk=2.2, 95% CI=1.99–2.52) than with COCs (relative risk=1.8, 95% CI=1.75–1.84). The risk period peaked at 6 months and declined thereafter. The same authors (
24) evaluated the risks for suicide attempts and completion in 15- to 33-year-old females followed for 8 years. Compared with women who never used hormonal contraceptives, the relative risk among users was twofold higher for attempts and threefold higher for suicides. An important finding was that the time course between hormonal contraceptive use and suicide attempts peaked after 2 months. This observation demonstrates the clinical relevance of monitoring mood symptoms and suicidality shortly after contraceptive initiation to identify vulnerable women. A similar Swedish study provided data on the association between hormonal contraceptive use and the first prescription for an anxiolytic, hypnotic, sedative, or antidepressant, or a depression diagnosis, in 12- to 20-year-old females (
25). In this cohort of nearly 25,000 individuals, 3.1% received prescriptions for psychotropic medications. The strongest association was for 12- to 14-year-old girls (odds ratio=3.46, 95% CI=3.04–4.94). The highest relative risk was observed for non-oral progestin-only methods in 12- to 14-year-olds (odds ratio=4.27, 95% CI=2.08–8.78). The relative risk declined with age, and no association was found for women over 21 years of age.
Contrasting findings have been reported by several investigators. A prospective study was conducted to track psychological symptoms in women after initiating DMPA or COCs compared with women who used nonhormonal contraception across a 2-year period (N=608) (
26). The longitudinal relationships between symptoms and contraceptive use were adjusted for age, number of visits, and baseline symptoms. COCs were protective against nervousness (odds ratio=0.5) and mood swings (odds ratio=0.7); DMPA was protective for mood swings (odds ratio=0.7) but was associated with weight gain.
A protective effect of hormonal contraceptive use for depression was also observed in women 25–34 years of age (
27). Contraceptive users were compared with sexually active women using either no contraception or nonhormonal contraception. CHC users had lower mean depressive symptom levels and were much less likely to report a past-year suicide attempt (odds ratio=0.37, 95% CI=0.14–0.95). The protective association with depressive symptoms was also found for women using progestin-only contraceptives. Similar findings were reported in the National Comorbidity Survey–Adolescent Supplement study (
28), which included adolescents who had never been pregnant. Current or lifetime OC users (including COCs and progestin-only pill preparations) were compared with non-OC users for the occurrence of depressive disorders. Although an elevated risk for lifetime depressive disorders among OC users was found in crude analyses, it was not significant in adjusted models (for ever OC users, adjusted odds ratio=1.10, 95% CI=0.88−1.37; for current OC users, adjusted odds ratio=1.00, 95% CI=0.77–1.29). Age and age at first intercourse were the variables that had the largest effects in elevating the crude odds ratio. Additional variables included in adjusted models were smoking history, body mass index, and socioeconomic status. No significant temporal relationship between OC use and depression was found.
In an important randomized placebo-controlled trial, women were randomized to a COC or placebo for three cycles. COC use was associated with small but statistically significant increases in mean anxiety, irritability, and mood swing scores during the intermenstrual phase; however, a significant improvement in depression was observed in the premenstrual period (
29). The proportion of women who reported clinically relevant mood deterioration did not differ between participants treated with a COC (24.1%) or those who received placebo (17.0%) (p=0.262). The authors concluded that subgroups of women suffer from COC-related side effects and others experience beneficial mood change in the premenstrual phase. The variability of women’s experiences limits generalizations about the impact of CHCs on psychiatric symptoms.
Successful treatment of disorders with hormonal contraceptives also has the potential to improve depressive symptoms. In a randomized placebo-controlled trial of adolescents treated with a COC or placebo for dysmenorrhea (
30), the mean Center for Epidemiology–Depression Scale (CES-D) score was used to assess change in depressive symptoms after 3 months. Among adolescents treated with a COC, the mean CES-D score was 16 (SD=9.9) at baseline and 14 (SD=9.2) at 3 months (p=0.26). In the placebo-treated group, the mean CES-D score was 17.8 (SD=11.6) at baseline and 14.4 (SD=8.1) at 3 months (p=0.06). While these decreases were not statistically significant, the observation that depressive symptom scores did not increase is reassuring. An interesting aspect of this study was that a subgroup of 11 women with high depressive symptom scores (CES-D score ≥27) who were treated with COCs improved (mean scores of 35.7 and 19.1 at baseline and study exit, respectively; p=0.003). Furthermore, the types and frequencies of side effects reported by adolescents treated with placebo were similar to those reported by adolescents treated with COCs.
The validity of the findings from any observational study depends on the degree to which confounding factors can be identified and included in statistical adjustment strategies. Confounding factors are associated with both the exposure (hormonal contraceptives) and the outcome (depression). For example, early sexual behavior is associated with smoking, alcohol consumption, and living in a nonintact family (
31). These factors are also associated with depression, which makes isolating the magnitude of the effect of the contraceptive method in adolescents challenging. These factors are often difficult to extract and quantify from the large databases used in all observational studies, and they act as unmeasured confounding variables.
Mechanisms of Hormonal Contraceptive Effects on Mood
The existence of a group of women who are particularly vulnerable to mood disturbance during hormonal fluctuations has been established (
32). Reproductive events in females—puberty, premenstruum, pregnancy, postpartum period, menopausal transition—are associated with an increased likelihood of psychiatric symptoms, most commonly depression. Affected women have normal peripheral concentrations of reproductive hormones, but their neurophysiology appears vulnerable to the mood-destabilizing effects of fluctuation in gonadal steroid concentrations. Whether these are the women who also are likely to develop depression with initiation of hormonal contraceptives has not been established.
The experience of females who have mood symptoms during CHC treatment has prompted investigators to consider explanatory mechanisms. Peripheral neurosteroids, which cross the blood-brain barrier, have been implicated. COCs have been shown to significantly decrease plasma neurosteroid concentrations by suppression of precursor steroids in healthy women without mood or anxiety disorders (
33); however, no change in psychiatric symptoms was observed. In one study, women with a history of mood deterioration induced by COCs participated in a double-blind randomized controlled trial to rechallenge them with COCs or placebo (
34). During the final week of treatment, COC users had higher scores for depressive symptoms, mood swings, and fatigue than placebo users. fMRI studies revealed changes in brain reactivity in regions associated with emotion processing and responsivity to ovarian steroid hormones. Another notable finding was that although all participants had previously experienced mood deterioration with COC use, only one-third of the women had mood worsening with COC treatment during the study.
The immune-regulating kynurenine pathway was evaluated in women treated with OCs compared with untreated women. A lower ratio of neuroprotective kynurenic acid to neurotoxic 3-hydroxykynurenine and quinolinic acid and a higher concentration of C-reactive protein was found in OC-treated compared with untreated women. The association between OC use and reduced kynurenic acid may contribute to the association between OC use and depression in vulnerable women (
35).
In a set of translational experiments, Graham and Milad (
36) demonstrated that estrogen facilitates the consolidation of fear extinction. Although treatment with COCs inhibits estrogen production, it had little or no effect on fear conditioning or acquisition of extinction, but extinction recall was significantly impaired. Diminished extinction memory may result in increased anxiety and reduce the effect of exposure therapy. The clinical significance of this finding in the general population of contraceptive users has not been established.
Contraception for Women with Psychiatric Disorders
In an analysis using data from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, which included a large representative sample of patients with major depression, postmenopausal women were categorized by contraceptive method (CHCs, N=232; a progestin-only formulation, N=58; no hormonal contraceptive, N=948) and compared on depressive symptoms (
37). Women receiving CHCs had less severe symptoms, better physical function, and a lower number of comorbid anxiety disorders, which the authors speculated might be associated with beneficial effects of ethinyl estradiol. The overall effects of hormone-based contraception on mood were small, and no evidence was found that any of these hormone treatments worsened depressive symptoms. Similarly, in a review of 17 double-blind placebo-controlled studies of fluoxetine treatment for major depression or bulimia (
38), the combination of OCs with fluoxetine compared to fluoxetine alone did not change depression scores significantly across the trial, affect the safety of either agent, or reduce the efficacy of contraception.
The authors of a systematic review (
39) concluded that that the use of OCs, levonorgestrel-IUD, and DMPA among women with depressive or bipolar disorders was not associated with worse psychiatric outcomes compared with women using no hormonal contraceptives. Women with bipolar disorder tolerated IUD well. Nearly 90% of women who chose a levonorgestrel- or copper-containing IUD continued use for at least 12 months (
40). For comparison, only 31% of those who initiated DMPA received the three additional scheduled injections within the next year. The rates of complications and psychiatric hospitalizations did not differ among women using IUD, DMPA, or sterilization methods.
In a study of women treated for opioid use disorder (N=260), unintended pregnancy was common (61.2%), and nearly half of the women did not use contraception (
41). Barriers to reproductive health services included concern about being judged, losing custody of children, cost, and transportation. The majority of participants expressed interest in receiving reproductive health services. However, women who have substance use disorders in addition to other psychiatric illnesses are less likely to receive prescription contraceptives than women without this comorbidity. In a study using the national Veterans Administration database assessing women with psychiatric disorders, substance use, both diagnoses, and neither diagnosis (
42), women with mental illness were as likely as women with no diagnosis to receive a prescription for contraception. In contrast, women with substance use disorders (with or without comorbid mental illness) were significantly less likely to receive a prescription for a contraceptive than women with neither diagnosis (
4).
Women with schizophrenia have more lifetime sexual partners and higher rates of coerced sex, unintended pregnancies, and abortions compared with women in the general population (
2). Symptoms of distractibility, disorganization, impulsive behavior, and substance use may compromise consistent or correct use of contraceptives. Limited education about sexuality, impulsivity, impaired cognitive function, and high rates of comorbid substance use contribute to poor overall health. Methods that do not require the performance of an action for optimal efficacy, such as LARCs, are preferable for women coping with difficult life circumstances. Women treated with long-acting depot antipsychotic drugs may also benefit from concomitant DMPA injections; however, we were unable to locate any publications about this practice. Adherence to psychotropic and contraceptive regimens is a challenge in both psychiatric and gynecologic practice.
Women with psychiatric disorders are likely to have other medical comorbidities that affect contraceptive recommendations. Estrogen-containing contraceptives increase the risk of cardiovascular events in women with hypertension and tobacco use. Tobacco use is more common in individuals with mental illness compared with the general population. The risk of cardiovascular morbidity with COC use in smokers increases with age (<35 versus >35 years) and magnitude of tobacco use. For older women who smoke >15 cigarettes a day, the health risk is unacceptable, according to the Medical Eligibility Criteria for Contraceptive Use (
6). Progestin-only or LARCs are the methods of choice for women with these conditions. Women with migraine with aura are at increased risk of stroke, and CHCs are contraindicated.
Psychotropic Drugs and Contraception
A recent review (
43) included studies on SSRIs (N=5), tricyclic antidepressants (N=4), bupropion (N=1), atypical antipsychotics (N=3), and benzodiazepines (N=5). Overall, no differences in unintended pregnancy rates were found for the combination of CHCs with or without psychotropic drugs, or conversely, in psychotropic drug treatment outcomes when administered with or without CHCs.
Although pharmacological interactions between psychotropic drugs and contraceptives are infrequent, important exceptions exist. Clozapine plasma concentrations increase when combined with hormonal contraceptives, including ethinyl estradiol and levonorgestrel, as a result of associated reductions in the activities of hepatic cytochrome P450 (CYP) 1A2, 2C19, and 3A4 enzymes (
44). Significant side effects such as hypotension, sedation, tremor, and nausea have been reported. Doses must parallel the contraceptive regimen and be reduced in the active hormone phase, when the clozapine plasma concentration may increase two- to threefold compared with the no-hormone phase. The IUD, implant, or DMPA, which do not undergo the first-pass hepatic metabolism of COCs, are preferable methods.
Ethinyl estradiol is a potent inducer of uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A4, and the plasma concentrations of drugs metabolized through this pathway are reduced. COC treatment decreases the serum concentrations of lamotrigine and valproic acid. In a study comparing the concentrations measured in the third week of active hormone administration with concentrations measured at the end of the week of inactive pill use, significant serum concentration reductions by 32.6% for lamotrigine and 23.4% for valproic acid were observed (
45). In a double-blind placebo-controlled crossover study in patients with epilepsy, women were treated with lamotrigine and randomized to treatment with a COC or placebo (
46). The mean serum lamotrigine concentration after placebo treatment was 84% higher than after COC treatment. Women with epilepsy have developed seizures during the 21-day ethinyl estradiol treatment period and toxicity during the hormone-free week; however, the effect of these rapidly changing lamotrigine concentrations on mood stability has not been determined. The 21-day ethinyl estradiol regimen creates fluctuating concentrations that can be avoided by treatment with continued monophasic COC treatment without an inactive pill period. Serum concentration measurements of lamotrigine should be performed after at least 7 days of ethinyl estradiol to achieve a steady-state value (
46).
Ethinyl estradiol also increases the clearance of valproic acid, primarily through multiple UGT enzymes. Treatment with valproic acid in reproductive-age women has been discouraged because of its association with reproductive endocrine disorders, including polycystic ovaries, elevated testosterone concentrations, and menstrual disorders. If an unintended pregnancy occurs, the embryo is at risk for major congenital malformations and later neurodevelopmental delays. When valproic acid is prescribed for women with psychiatric disorders, highly effective contraception methods, such as LARCs, are recommended.
Lamotrigine and carbamazepine induce the production of sex hormone–binding globulin, which tightly binds progestins, reduces the concentration of free progestin, and increases the risk of contraceptive failure (
47). Carbamazepine and oxcarbazepine induce the CYP3A4 system and increase the clearance of contraceptive steroids, which may result in unintended pregnancy. Plasma concentrations of ethinyl estradiol and levonorgestrel have been found to be lower by about 50% in carbamazepine-treated women compared with nontreated women (
48). CHCs and progestin-only pill preparations are not recommended for women being treated with carbamazepine. The effectiveness of the transdermal patch and vaginal ring also is reduced in women treated with carbamazepine. The subdermal implant is acceptable, and the IUD (copper-containing or locally active levonorgestrel) and DMPA are recommended.
The use of herbal products for depression is common in reproductive-age women; however, meager data are available with respect to interactions with COCs. Compounds such as St. John’s wort induce CYP3A4, the primary hepatic enzyme that metabolizes ethinyl estradiol (
49). Pharmacokinetic studies of treatment with St. John’s wort combined with COCs have yielded mixed results but suggest weak to moderate induction of COC metabolism, an increased risk for breakthrough bleeding, and significantly lower hormone concentrations, which theoretically may compromise efficacy (
50).
Conclusions
Depression is a common disorder in reproductive-age women for whom contraceptives are prescribed. The magnitude of association between contraceptive use and depression is variable across studies, with reports of substantial benefits and risks. The variability in these largely observational studies is due to multiple factors, including differences in sampling frames, sociocultural factors, population characteristics, and the inevitable confounding variables. Randomization, which distributes unknown, unmeasured variables that affect the outcomes between groups being compared, is not available in observational studies.
Although variability in this literature is common, clinical studies and randomized placebo-controlled trials of women with psychiatric disorders have generally reported similar or lower rates of mood symptoms in hormonal contraceptive users compared with nonusers. However, it is clear that some women who are treated with hormonal contraceptives will develop dysphoric mood. Although a causal contribution to symptom emergence has not been established, the clinical relevance of these findings is that the response of an individual woman to hormonal contraceptive treatment is not predictable. Although the risk is low, the potential for the development of psychiatric symptoms must be included in the discussion of the CHC risk profile, which provides an opportunity for instruction. Education of childbearing-age women about depression is recommended by ACOG, and screening for depressive disorders is an essential component of well-woman care. The Patient Health Questionnaire–9 is a common screening tool available in most electronic medical records. Establishing a pre-contraceptive measurement of depressive symptoms provides a quantitative measure for comparison across time. Early postinitiation monitoring for side effects provides an opportunity for early intervention and continued use of effective contraception. The goal is to increase the likelihood that our patients will achieve their mental and reproductive health goals.
The majority of cited publications have focused on harms (particularly depression) that may be associated with CHC use. Quantitative evaluation of the benefits to be balanced against harms is not explored in most investigations. Important and unaddressed questions are whether antidepressant treatment prevents the emergence of mood symptoms in women who begin taking a hormonal contraceptive agent, and the rate at which (or whether) CHC-emergent depression responds to pharmacotherapy or psychotherapy. Research to explore risk factors that identify the vulnerable group of women who are sensitive to hormonal contraceptives would be an important advance in individualizing care. Simple and clear communication methods to address the sexual health and contraceptive needs of women with serious mental illness are needed. An example of combined services would be the development of a protocol for the coadministration of DMPA with depot antipsychotic drugs.
We assert that basic contraceptive knowledge is a critical component of our care of female patients and that residency and continuing education programs must include this important topic. Unfortunately, in a survey of medical residents in several specialties (N=768), respondents reported having received limited training about prescribing contraceptives for patients with severe and persistent mental illness (
51). By specialty, 30.6% of the residents were from family medicine, 18.1% from obstetrics/gynecology, 10.8% from internal medicine, and from 10.4% psychiatry; 30.1% did not indicate a specialty. The majority of residents (60%) disagreed or strongly disagreed that they had received such training. A newly available resource, the National Curriculum in Reproductive Psychiatry, which is free and funded by the American Board of Psychiatry and Neurology (
http://ncrptraining.org/), is available to address this information gap. Comprehensive training is also available through the Family Planning National Training Center (
http://www.ctcfp.org/category/contraception/), funded by the Department of Health and Human Services Office of Population Affairs.
Acknowledgments
The authors thank Emily S. Miller, M.D., M.P.H., Crystal T. Clark, M.D., M.Sc., and Katie Watson, J.D., for their comments on drafts of the manuscript. The authors also appreciate the efforts of Barbara Sutcliffe in organizing the references across multiple drafts.