Long-acting injectable (LAI) antipsychotics allow for a complete tracking of adherence and decrease the risk of misuse (
1–
4). Although they can be perceived as a last-resort option (
5,
6), a broader and earlier use of LAIs has been emphasized in recent evidence-based guidelines (
7–
10), mainly based on the growing evidence of their effectiveness in preventing relapse and rehospitalization (
2,
3,
11,
12), the well-established data on the negative consequences of poor adherence during the early phases of psychosis (
13,
14), and their possible role in relieving the daily burden of oral antipsychotic administration (
15,
16). However, although existing guidelines consider LAIs to be an important option for the maintenance treatment of schizophrenia, they do not provide any clear suggestion on which should be considered as first-choice options. Pragmatically, the U.K. National Institute for Health and Care Excellence recommends the use of the same criteria that are applied for the choice of oral antipsychotics (
7), but this guidance does not consider practical differences in the administration modalities of individual LAIs that may account for different efficacy and acceptability profiles (e.g., long compared with short intervals of administration, the need for oral supplementation in the first few weeks of LAI administration, the need for monitoring after administration, or local pain) (
1). Further, pharmacokinetic and pharmacodynamic differences between oral and LAI formulations may account for different efficacy and tolerability profiles (
4,
17). Existing systematic reviews and meta-analyses have focused mainly on the comparison between oral and LAI antipsychotics, generally sorted into broad and heterogeneous groups (
2,
3,
18,
19), without considering the different clinical profiles of the various LAIs. Randomized controlled trials comparing two or more LAIs in people with schizophrenia and other nonaffective psychosis have provided conflicting results (
20,
21). Based on these considerations, we conducted a network meta-analysis to assess the differential efficacy and acceptability of individual LAIs among people with nonaffective psychoses.
Methods
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines specific for network meta-analysis (
22) (see Supplement A in the
online supplement). The study protocol was registered in advance in PROSPERO (International Prospective Register of Systematic Reviews; registration number: CRD42019120240).
Study Selection and Data Extraction
We searched for randomized controlled trials that included adults (≥18 years old) who were diagnosed with a nonaffective psychotic disorder according to any validated diagnostic criteria and who required antipsychotic maintenance treatment. No time or language restrictions were applied. All available LAIs, according to the Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization (WHO) (
https://www.whocc.no/atc_ddd_index), were eligible. Although the ultimate goal of this review was to compare LAIs with one another, we also included studies comparing LAIs with placebo and with oral antipsychotics to develop a more informative network of comparisons. First-generation oral antipsychotics were grouped according to chemical classes as defined by the ATC. We excluded studies comparing oral antipsychotics head to head or against placebo, considering clinical differences relative to LAI randomized controlled trials and also considering the risk of violating the transitivity assumption required for network meta-analyses (
23). Studies comparing LAIs with a mixture of oral antipsychotics were also excluded. Finally, as relapse was a primary outcome, we excluded randomized controlled trials lasting <12 weeks, as has been suggested (
24).
We searched the electronic databases MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Trials (CENTRAL), and CINAHL; online trial registers (e.g., ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform); and databases of regulatory agencies and pharmaceutical companies. We searched records from database inception to June 8, 2020 (for the full search strategy, see Supplement B in the
online supplement). Two of us (G.O., F.B.) independently assessed titles, abstracts, and full texts of potentially relevant articles and extracted data following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (
25). Two of us (F.B., C.G.) assessed the methodological quality of included studies using the Cochrane risk of bias tool. Disagreements were resolved by discussion and consensus with a third author (C.B.).
Outcomes
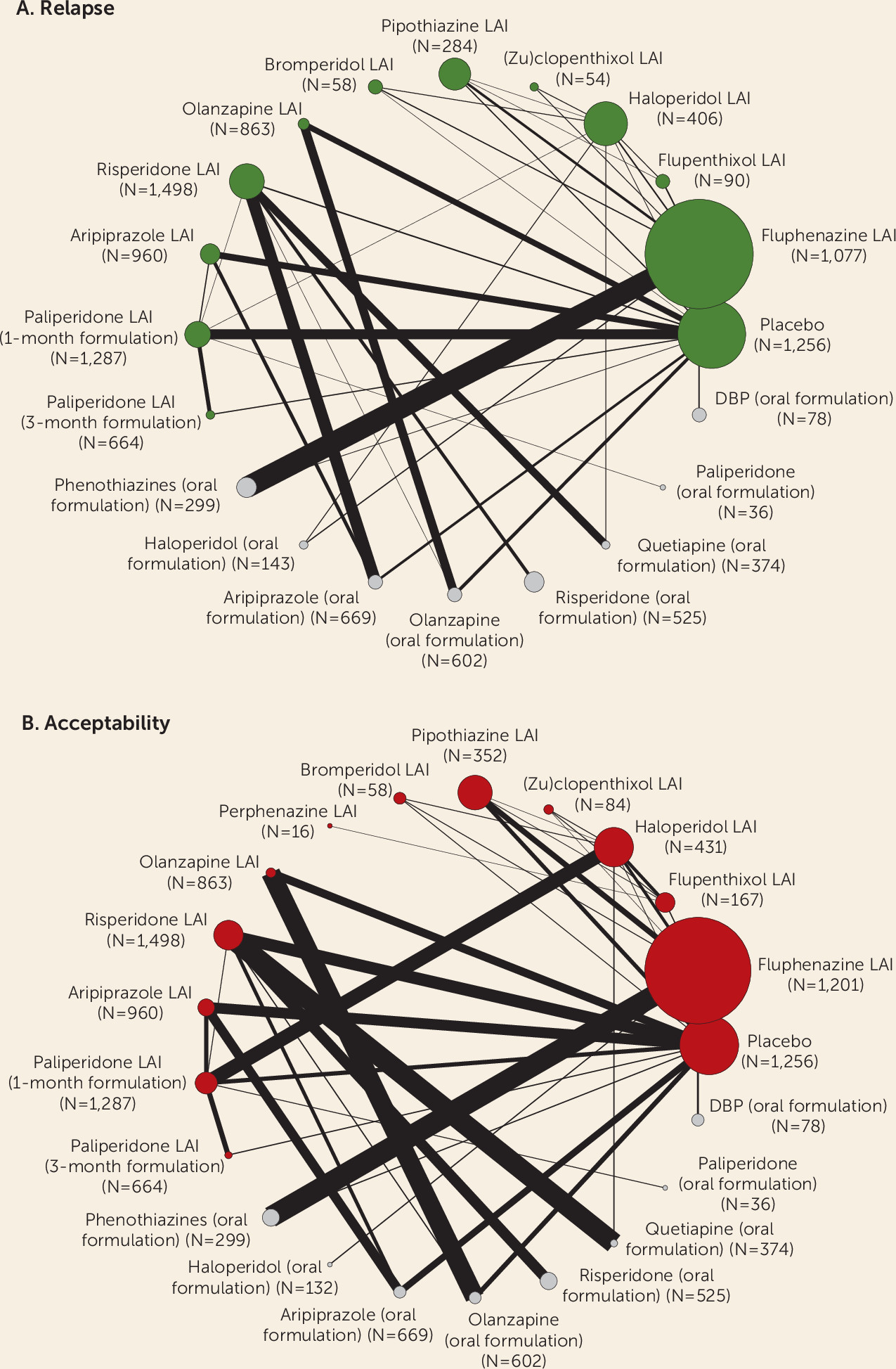
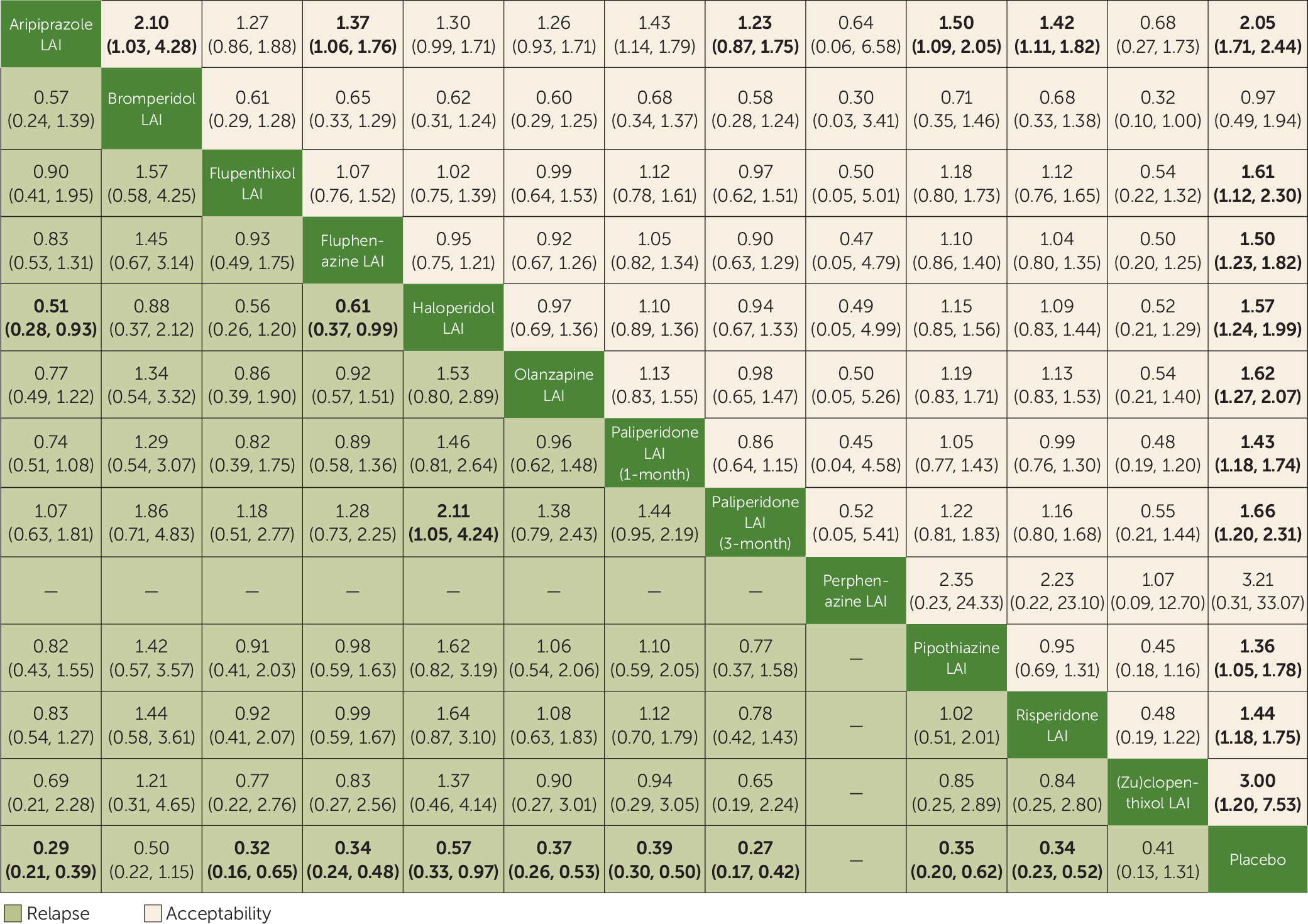
Two primary outcomes were considered: the number of patients who experienced at least one study-defined relapse by the end of the trial, as a proportion of the total number of patients who underwent randomized assignment (indicated as “relapse”); and the number of patients who dropped out by the end of the trial for any cause, as a proportion of the total number of patients who underwent randomized assignment (indicated as “acceptability”).
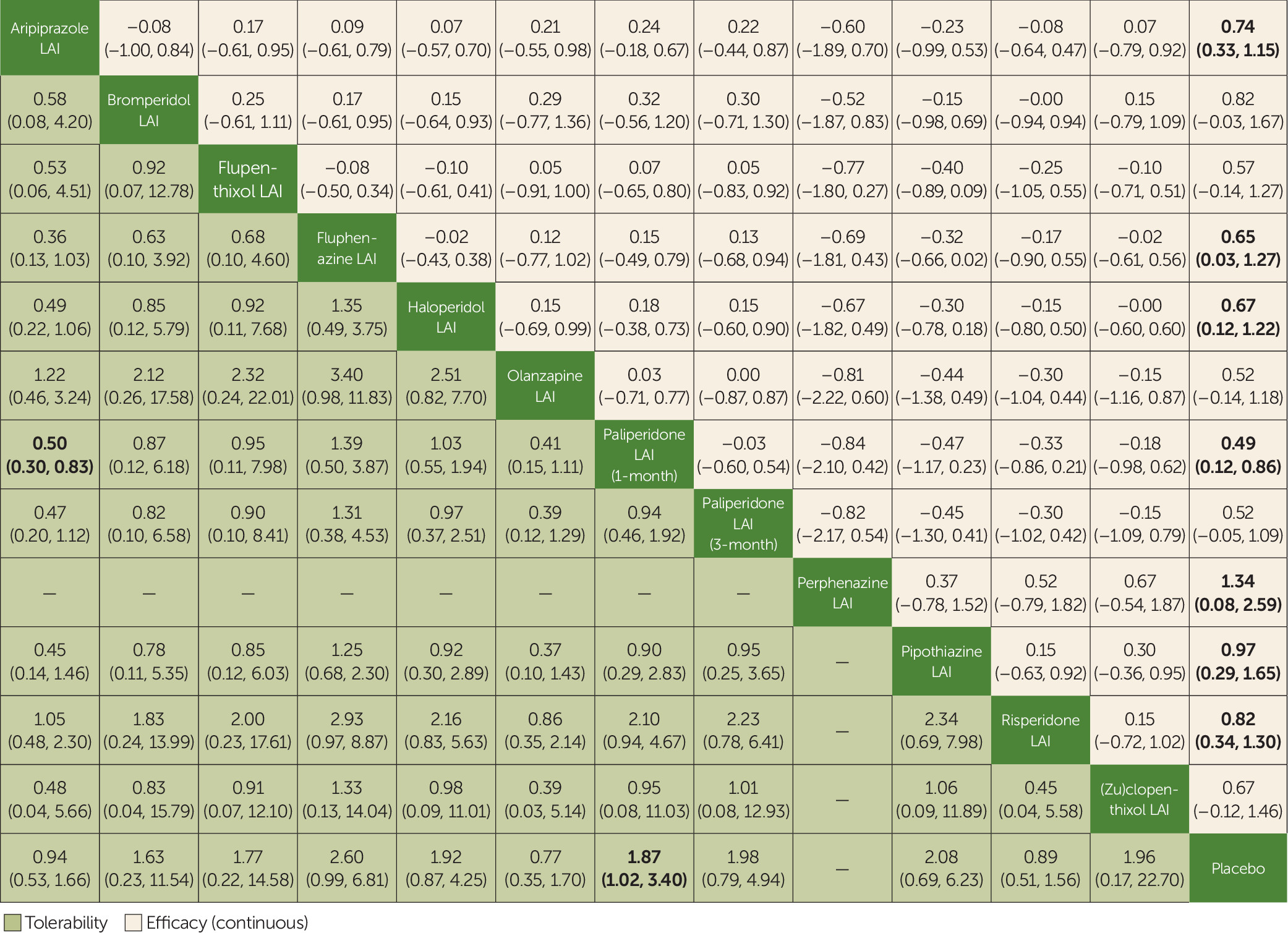
Secondary outcomes included the mean change in scores on validated rating scales measuring psychopathology at study endpoint (“efficacy”), the number of patients who dropped out by study endpoint because of any adverse event (“tolerability”), and the mean change in scores on validated rating scales measuring quality of life at the end of the trial. Additional secondary outcomes, not included in the original protocol, were analyzed to provide further results on efficacy and side effects and were regarded as merely exploratory. These included functioning, hospitalization, sedation, QTc prolongation, weight gain, hyperprolactinemia, and extrapyramidal symptoms.
Statistical Analysis
We performed a standard pairwise random-effects meta-analysis for every comparison and, for each outcome, a network meta-analysis with a random-effects model in a frequentist framework, using the Stata
mvmeta package. For dichotomous outcomes, we calculated and pooled relative risks with 95% confidence intervals. For continuous outcomes, we pooled the mean differences between treatment arms at the end of the study if all trials used the same rating scale; otherwise, we pooled standardized mean differences. We calculated dichotomous data on a strict intention-to-treat basis, considering as the denominator the total number of patients who underwent random assignment. For continuous variables, we applied a modified intention-to-treat analysis, whereby participants with at least one postbaseline measurement were represented by their last observations carried forward. The two primary outcomes were tested independently, without applying correction for multiple testing, as recommended by the Cochrane Handbook (
26).
When a study included different arms of the same antipsychotic (LAI or oral) at different doses, we pooled these arms into a single one (
25), provided that they were administered within a therapeutic dose range (
27,
28). Very low doses of antipsychotics were considered as pseudo placebo, as endorsed by regulatory agencies (
29), and were pooled together with placebo in the analysis. Furthermore, considering their pharmacological similarity (
30), fluphenazine enanthate and decanoate, as well as clopenthixol and zuclopenthixol decanoate, were pooled together.
We asked trial authors to supply missing data or, alternatively, we imputed data with validated statistical methods (
25). We calculated missing standard deviations based on the standard error, t statistics, or p values (
31). If this was not possible, we substituted missing standard deviations with a mean of those reported in the other included trials (
32). As a last option, we used the standard deviation of the mean baseline score. Missing data for relapse were imputed according to commonly used cutoff scores of validated rating scales (namely, an increase ≥25% on scores on the Positive and Negative Syndrome Scale [PANSS], an increase ≥30% on scores on the Brief Psychiatric Rating Scale [BPRS], and an increase ≥2 points on scores on the Clinical Global Impressions severity scale [CGI-S]) (
33–
35), using a validated methodology (
36).
For pairwise meta-analyses, we assessed heterogeneity by visual inspection of forest plots and by I
2 statistics. For the network meta-analysis, common heterogeneity across all comparisons was assumed and estimated in each network (
37).
We evaluated the assumption of transitivity by extracting potential effect modifiers (e.g., blinding, sample size, follow-up length, antipsychotic doses) and comparing their distribution across comparisons in the network.
We evaluated the presence of incoherence by comparing direct and indirect evidence within each closed loop (
38) and comparing the goodness of fit for a network meta-analysis model that assumes consistency with a model that allows for incoherence in a design-by-treatment framework (
39–
41), using the Stata commands
mvmeta and
ifplot (
42,
43) and the Stata network suite (
44). Incoherence was further investigated through node-splitting (
45) and side-splitting (
44) approaches between comparisons.
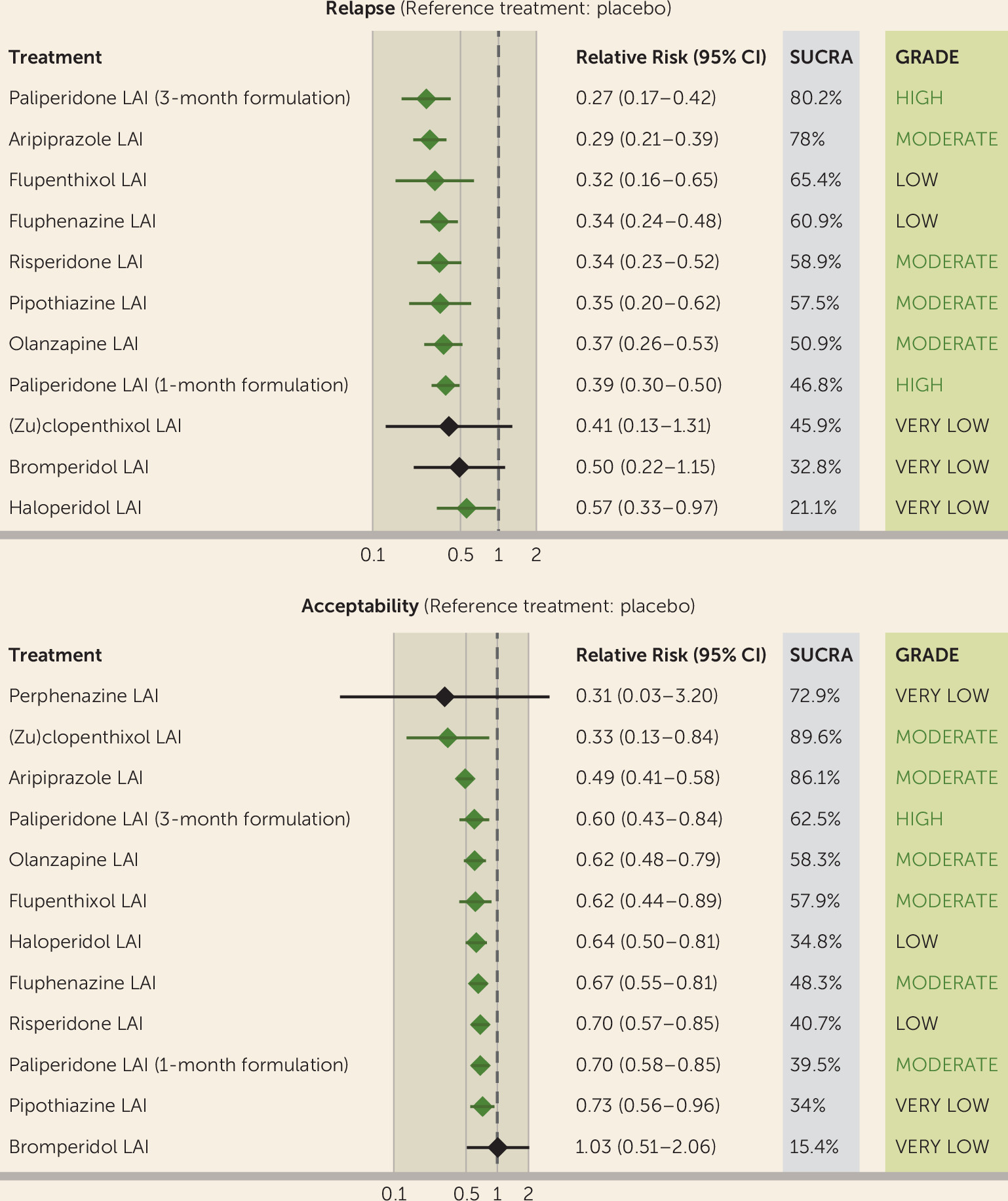
For the primary outcomes, we produced a treatment hierarchy by means of surface under the cumulative ranking curve (SUCRA) and mean ranks (
46).
If ≥10 studies were included in a primary outcome, we assessed publication bias by visually inspecting the funnel plot, testing for asymmetry with the Egger’s regression test (
47), and investigating possible reasons for funnel plot asymmetry.
For each primary outcome, we assessed the certainty of evidence from network meta-analyses through the CINeMA application (
https://cinema.ispm.ch), an adaptation of the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) (
23,
48).
Finally, for each primary outcome, we conducted four sensitivity analyses excluding trials that did not employ a double-blind design; trials that compared LAIs with placebo; trials that involved ≤50 participants and were published before 1990; and trials that had a high risk of bias (i.e., ≥3 risk of bias items at “high risk”).
Discussion
In this network meta-analysis, most LAIs were superior to placebo in preventing relapse and were significantly more acceptable than placebo. For both of these primary outcomes, most LAIs had fairly similar effect sizes, and no relevant differences emerged when they were compared head to head, except for aripiprazole, which performed particularly well against other LAIs regarding acceptability. Importantly, the certainty of evidence was moderate or high for a number of LAIs, particularly second-generation LAIs. However, only the 3-month formulation of paliperidone, the 1-month formulation of paliperidone, aripiprazole, and olanzapine were supported by a moderate to high certainty of evidence in both primary outcomes and therefore can be regarded as reasonable first-line maintenance treatments in people with schizophrenia and related nonaffective psychosis. In general, these findings were confirmed by secondary analyses, such as efficacy measured via rating scales, hospitalization rates, and tolerability outcomes, although data on quality of life and functioning were lacking. The 3-month formulation of paliperidone and aripiprazole LAI were among the best performing treatments against placebo in many analyses and had very high SUCRA rankings in both primary outcomes.
The analysis of individual adverse events, although limited by relatively few and heterogeneous data, confirmed that weight gain and hyperprolactinemia may be relevant also for those LAIs with good overall acceptability and tolerability (i.e., paliperidone [1- and 3-month formulations], aripiprazole, and olanzapine).
The findings of this network meta-analysis are consistent with those from the largest randomized trials comparing LAIs head to head (
106,
112,
118) and with large observational studies that analyzed the efficacy of individual LAIs in preventing rehospitalization (
11,
135).
To our knowledge, this is the first comparison of individual LAIs using a network meta-analysis methodology. This approach allowed comparisons of LAIs for which no direct evidence was available while avoiding questionable pooled subgroups (i.e., first- or second-generation LAIs) and obtaining more precise estimates. The primary analyses included more than 11,000 participants, making this the largest meta-analysis conducted on LAIs to date. Furthermore, estimates for dichotomous outcomes are conservative, as they were calculated considering the total number of patients who underwent randomized assignment in the denominator.
Despite these strengths, several limitations should be considered when interpreting the results. First, although we aimed to evaluate the ability of LAIs in preventing relapse in patients already stabilized, for some randomized controlled trials, stabilization was not clearly described. Therefore, several factors were considered as proxy measures of stabilization (e.g., the PANSS score at recruitment), which may lack precision. Second, included randomized controlled trials were published across a long time span and therefore are heterogeneous in terms of methodology, diagnostic criteria, follow-up periods, and outcomes. Despite that, overall coherence appeared to be well preserved for most analyses. Third, for a relevant number of studies, important information was lacking, and imputation methods had to be employed. Although this is an acceptable approximation in most cases (
32), some degree of imprecision cannot be excluded. Fourth, placebo-controlled studies may suffer from intrinsic limitations (
20,
136), in particular, the selection of relatively well-stabilized patients. The sensitivity analyses that removed these studies confirmed that placebo-controlled studies may have introduced some overall heterogeneity and incoherence, although overall results did not change substantially. Placebo-controlled studies of paliperidone (3-month formulation) may be of particular concern, considering that patients in these studies underwent a stabilization phase with paliperidone (1-month formulation) before randomization to the 3-month formulation or placebo. This study design may have inflated the effect size of the 3-month formulation of paliperidone by using a particularly enriched sample for benefit and tolerability in patients ultimately randomized to the placebo discontinuation phase of the study. Fifth, risk of bias was relatively high for many studies, particularly regarding attrition, reporting, and sponsorship biases. However, a sensitivity analysis showed that primary outcomes did not relevantly change after removing these studies. Sixth, some secondary outcomes, such as quality of life, functioning, and common adverse events, which might play a relevant role in helping clinicians to tailor LAIs to individual patients, were poorly reported by the original studies, leading to poorly populated and connected networks, high imprecision, and heterogeneity. These outcomes were not originally included in the protocol and were regarded as merely exploratory. Nevertheless, a meta-analysis found that LAIs and their corresponding oral antipsychotics did not differ significantly in 97% of the 119 analyzed adverse effects (
137). Seventh, as no comparison included ≥10 studies, the risk of publication bias could not be ruled out. Considering that we included only one unpublished trial (
87) and that many data from old studies were included, publication bias cannot be completely excluded, although it is expected to be less relevant compared with studies of other classes of psychotropic drugs (
138). Lastly, the network meta-analytic approach is not free from technical and theoretical shortcomings, including the risk related to multiple statistical assumptions and the challenges in addressing the problem of intransitivity and incoherence (
139).
The findings of this network meta-analysis have relevant implications for policy and research. Current guidelines emphasize the importance of considering LAIs for maintenance treatment of patients who might prefer this formulation for practical reasons, that is, those in the earliest illness phases and those with adherence problems (
7–
10). However, no clear indication is provided on which LAIs should be considered the first-choice options. Guidelines from the U.K. National Institute for Health and Care Excellence suggest following the same criteria used for oral antipsychotics, but it is unclear whether the efficacy and tolerability of the two formulations are identical (
4,
11,
135,
137,
140), and results from this network meta-analysis suggest that LAI characteristics, such as the time between administrations, might play a relevant role. Results from this study can help clinicians in tailoring the choice of LAI even from the first episode of psychosis, considering the impact of a successful maintenance treatment on long-term outcomes (
141). From a global health standpoint, it is relevant to consider that the WHO Model List of Essential Medicines (
142) includes only fluphenazine as an LAI formulation, although this medication is no longer regularly supplied globally, causing a disservice for the most vulnerable populations (i.e., those in low- and middle-income countries and humanitarian settings). Therefore, we argue that results from this network meta-analysis should rapidly inform the update of guidelines from the WHO and other organizations, with the aim of informing and improving psychiatric care worldwide.
Large, pragmatic, and high-quality head-to-head studies comparing LAIs are needed to overcome the methodological limitations mentioned above, including the lack of information on functioning, quality of life, common adverse events, and cost-effectiveness. Further, studies recruiting patients after the first episode of psychosis are needed to confirm the clinical utility of LAIs when utilized from the earliest phases of the disease, reversing the paradigm of LAIs as treatments reserved for patients with the most severe and chronic forms of illness.