Major depressive disorder (MDD) is a complex and often disabling mood disorder with an estimated lifetime prevalence of 20.6% in the United States (
1). Despite the availability of many pharmacological treatment options, including selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, results from a World Mental Health Survey showed that among patients treated for MDD, only 41% received minimally adequate treatment (
2). Effective treatment of MDD remains a clinical challenge, as nearly half of patients treated with standard first-line antidepressant monotherapy do not adequately respond (
3,
4). The Sequenced Treatment Alternative to Relieve Depression trial showed that only one-third of patients with MDD achieved remission with first-line monotherapy, and with successive treatment failures, patients were increasingly less likely to respond to subsequent treatment (
5). An insufficient response to an adequate course of treatment remains a critical problem in the management of patients with MDD.
Current pharmacological strategies to treat patients who do not respond to first-line antidepressant monotherapy include switching antidepressants (either within or between classes), combination therapy in which multiple standard antidepressants are used simultaneously, and augmentation of ongoing antidepressant monotherapy with adjunctive medications such as mood stabilizers or atypical antipsychotics (
6). Some atypical antipsychotics have demonstrated efficacy as adjunctive treatment for MDD (
7,
8). At present, cariprazine, aripiprazole, extended-release quetiapine, and brexpiprazole are the only adjunctive treatments approved by the U.S. Food and Drug Administration (FDA) for MDD (
9). In the VA Augmentation and Switching Treatments for Improving Depression Outcomes clinical trial, significantly higher remission rates were achieved with atypical antipsychotic augmentation (i.e., ongoing antidepressant treatment plus aripiprazole) compared with switching antidepressants (i.e., switch to bupropion) but not compared with combination treatment (i.e., another antidepressant plus bupropion) (
10). Response rates were significantly higher for patients who augmented with aripiprazole than for patients who switched or combined antidepressants. Additionally, a meta-analysis of studies in patients with MDD and inadequate response to standard antidepressant treatment found that, compared with adding placebo to standard antidepressant treatment, adding an atypical antipsychotic increased the probability of response and remission by 35% and 75%, respectively; those receiving atypical antipsychotic augmentation were, however, more likely to discontinue treatment due to adverse events compared with those receiving placebo augmentation (
8).
Cariprazine is a dopamine D
3-preferring D
3/D
2 and serotonin 5-HT
1A receptor partial agonist that is FDA approved for the treatment of adults with schizophrenia, with manic, mixed, or depressive episodes of bipolar I disorder, and as adjunctive therapy for the treatment of MDD (
11). Although the exact mechanism of antidepressant activity is unknown, the pharmacological profile of cariprazine may play a role in its efficacy and tolerability profile. Compared with other antipsychotics, cariprazine exhibits a greater affinity for and higher occupancy of D
3 receptors (
12,
13), which are highly expressed in brain regions involved in cognitive function, motivation, and reward-related behavior. This profile supports the hypothesis that pharmacological engagement of D
3 receptors by cariprazine may positively affect cognition, mood, or measures of reward, including reduction in anhedonia (
14–
18). Cariprazine also acts on other receptors that are suggested to have antidepressant effects, most notably through 5-HT
1A partial agonism, which may enhance the effects of serotonin reuptake inhibitors (
19). Cariprazine has two active metabolites, desmethyl cariprazine and didesmethyl cariprazine; the combined half-life of the three active moieties is approximately 1 week (
20). Therefore, changes in cariprazine dosage will not be fully reflected in plasma for several weeks, suggesting the potential for a longer time to relapse (
21), as well as delayed adverse reactions and treatment response after initiating treatment or adjusting the dosage (
11).
A previously published placebo-controlled flexible-dose study found that adjunctive cariprazine at 2.0–4.5 mg/day was more effective than placebo in improving depressive symptoms in adults with MDD (
22). Two additional flexible-dose cariprazine studies have been published in which reductions in depressive symptoms were numerically, but not significantly, greater for most evaluated cariprazine dosages compared with placebo; a fractional 0.1–0.3 mg/day cariprazine dosage was not therapeutically effective (
23,
24). The present fixed-dose study assessed the efficacy, safety, and tolerability of adjunctive cariprazine at 1.5 mg/day and 3.0 mg/day compared with placebo in the treatment of adults with MDD and inadequate response to antidepressant treatment alone.
Methods
This phase 3 study was conducted from November 2018 to September 2021 at 116 study centers in the United States, Bulgaria, Estonia, Germany, Hungary, Ukraine, and the United Kingdom. Participants were screened and recruited in compliance with the International Conference on Harmonization Good Clinical Practice guideline and the Declaration of Helsinki. The study was approved by institutional review boards (U.S. sites) or ethics committees and government agencies (European sites). Prior to study initiation, all participants provided written informed consent after receiving a complete description of the study that included the possibility of being assigned to one of two active-treatment groups or a placebo group. An interactive web-based system was used to randomize participants; all patients and investigators were blinded to treatment allocation throughout the study. Ratings and assessments of efficacy scales were performed by trained and certified raters at the study site; only medically qualified raters could administer assessments of extrapyramidal symptoms. Investigational product and placebo oral capsules were identical in appearance and taken at approximately the same time each day.
Study Design
This was a randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study of cariprazine as an adjunct to antidepressant treatment in adults with MDD. A 1- to 2-week screening period (with up to an additional 7 days if needed) that included washout of prohibited psychotropic medications, except for one ongoing antidepressant treatment, was followed by a 6-week double-blind treatment period in which patients continued taking the same antidepressant at the same dosage they were taking at baseline. After double-blind treatment, patients entered a 4-week safety follow-up (no study medication was taken during this time). Patients were randomized in a 1:1:1 ratio to receive placebo, cariprazine at 1.5 mg/day, or cariprazine at 3.0 mg/day. All participants randomized to cariprazine began on 1.5 mg/day; those in the cariprazine 1.5 mg/day group remained at that dosage, while those in the cariprazine 3.0 mg/day group received 1.5 mg/day for 2 weeks, followed by an increase to 3.0 mg/day on day 15. Drug holidays up to 3 consecutive days were allowed if tolerability issues occurred with the allocated fixed dosage.
Participants
Participants were outpatients 18–65 years of age who met DSM-5 criteria for MDD, confirmed by the administration of the Structured Clinical Interview for DSM-5 (
25). Included patients had a current major depressive episode with a duration ≥8 weeks and <24 months and an inadequate response (<50% improvement) to one to three antidepressant treatment courses of adequate dosage and duration (at or above the minimum dosage per package insert for ≥6 weeks, with ≥3 weeks above the minimal dosage), as measured by a modified version of the Antidepressant Treatment Response Questionnaire (
26). At screening and baseline, a minimum score of 22 on the 17-item Hamilton Depression Rating Scale (HAM-D) (
27) and a score of ≥2 on item 1 (depressed mood) of the HAM-D were required. The SAFER criteria inventory (
28) was administered by an independent rater at screening to confirm that patients who met study inclusion and exclusion criteria had acute symptoms that were appropriate and valid for the trial. Patients had normal physical examination, clinical laboratory, and ECG results or clinically insignificant abnormal results (investigator judged).
Patients were excluded if they had one or more of the following: Young Mania Rating Scale (YMRS) (
29) score ≥12; current psychiatric diagnosis other than MDD (including intellectual disability or an anxiety disorder other than specific phobia); any substance use disorder in the previous 3 months; suicide risk or risk of injury to self or others; and history of nonresponse to more than three antidepressant trials of adequate dosage (in the context of the current major depressive episode). Patients with a history of specific treatments (i.e., esketamine, ECT, vagus nerve stimulation, or transcranial magnetic stimulation) during the current episode or within 6 months of screening were excluded. Initiation or termination of psychotherapy for depression within 3 months of screening or plans to initiate, terminate, or change such therapy during the study were also exclusionary. Patients taking stable doses of benzodiazepines could continue use during the study. Other psychotropic drugs were prohibited, except for the ongoing antidepressant treatment and zolpidem, zaleplon, eszopiclone, zopiclone, chloral hydrate, or suvorexant for insomnia; episodic use of lorazepam for agitation, restlessness, or hostility; and benztropine, biperiden, diphenhydramine, trihexyphenidyl, or propranolol for extrapyramidal symptoms or akathisia.
Efficacy Outcomes
The primary and secondary efficacy parameters were change from baseline to week 6 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score (
30) and Clinical Global Impressions severity (CGI-S) score (
31), respectively (assessed at screening, baseline, and weeks 1, 2, 4, and 6). Additional efficacy parameters (assessed at screening, baseline, and at least one double-blind visit) included MADRS response (reduction ≥50% in MADRS total score) and remission (MADRS total score ≤10); change from baseline in HAM-D total score and Hamilton Anxiety Rating Scale (HAM-A) total score (
32); and Clinical Global Impressions improvement (CGI-I) score and response (CGI-I score ≤2).
Safety Outcomes
Treatment-emergent adverse events, vital signs, and suicide risk (Columbia-Suicide Severity Rating Scale score [
33]) were recorded at every visit. ECG results, physical examination, and clinical laboratory monitoring were evaluated at screening and at the end of week 6. Treatment-emergent mania was assessed by YMRS scores at screening, baseline, and week 6. Extrapyramidal symptoms were assessed as treatment-emergent adverse events and by rating scale scores (Barnes Akathisia Rating Scale [
34], Abnormal Involuntary Movement Scale [
31], and Simpson-Angus Scale [
35]) at baseline and weeks 1, 2, 4, and 6.
Statistical Analysis
Safety assessments were based on the safety population (randomized patients who took at least one dose of investigational product); efficacy assessments were based on the modified intent-to-treat (mITT) population (randomized participants who took at least one dose of study drug and had at least one postbaseline MADRS measurement). The primary efficacy parameter (change in MADRS total score from baseline to week 6) was analyzed by a mixed-effects model for repeated measures (MMRM) with treatment group, country, antidepressant treatment failure category, visit, and treatment group-by-visit interaction as fixed effects and the baseline value and baseline-by-visit interaction as covariates. An unstructured covariance matrix was used to model the covariance of within-patient scores, and the Kenward-Roger approximation was used to estimate denominator degrees of freedom. Sensitivity analyses for the primary endpoint using the pattern-mixture model and copy-reference approach were also performed. The results were consistent with the primary analysis. Analyses of changes from baseline in CGI-S score were conducted with an MMRM similar to the model used in the primary efficacy analysis.
By-visit changes from baseline were analyzed with an MMRM, as well as with an analysis of covariance model with last observation carried forward (LOCF) imputed for MADRS total score, CGI-S score, HAM-A total score, and HAM-D total score with treatment group, country, and antidepressant treatment failure category as factors and baseline values as covariates. MADRS and HAM-A response and remission rates were analyzed by a logistic model (with LOCF imputation) with treatment group, country, antidepressant treatment failure category, and baseline score as explanatory variables.
Sample size was determined with an assumed effect size of 0.286 for the primary endpoint; it was determined that 250 participants per treatment group would provide approximately 90% power to detect that at least one cariprazine dosage was statistically significant with multiplicity adjustment compared with placebo.
The two-stage mixture parallel gatekeeping procedure (truncated Hochberg with truncation parameter of 0.9 for the primary endpoint and regular Hochberg for the secondary endpoint) was used to control the overall type I error rate at a 0.025 level (one-sided) for multiple comparisons of two active dosages with placebo.
Safety parameters were analyzed descriptively. Treatment-emergent mania (YMRS score ≥16) and treatment-emergent extrapyramidal symptoms (parkinsonism: Simpson-Angus Scale score ≤3 at baseline and >3 at any postbaseline visit; akathisia: Barnes Akathisia Rating Scale score ≤2 at baseline and >2 at any postbaseline visit) were assessed.
Results
Patient Disposition and Baseline Characteristics
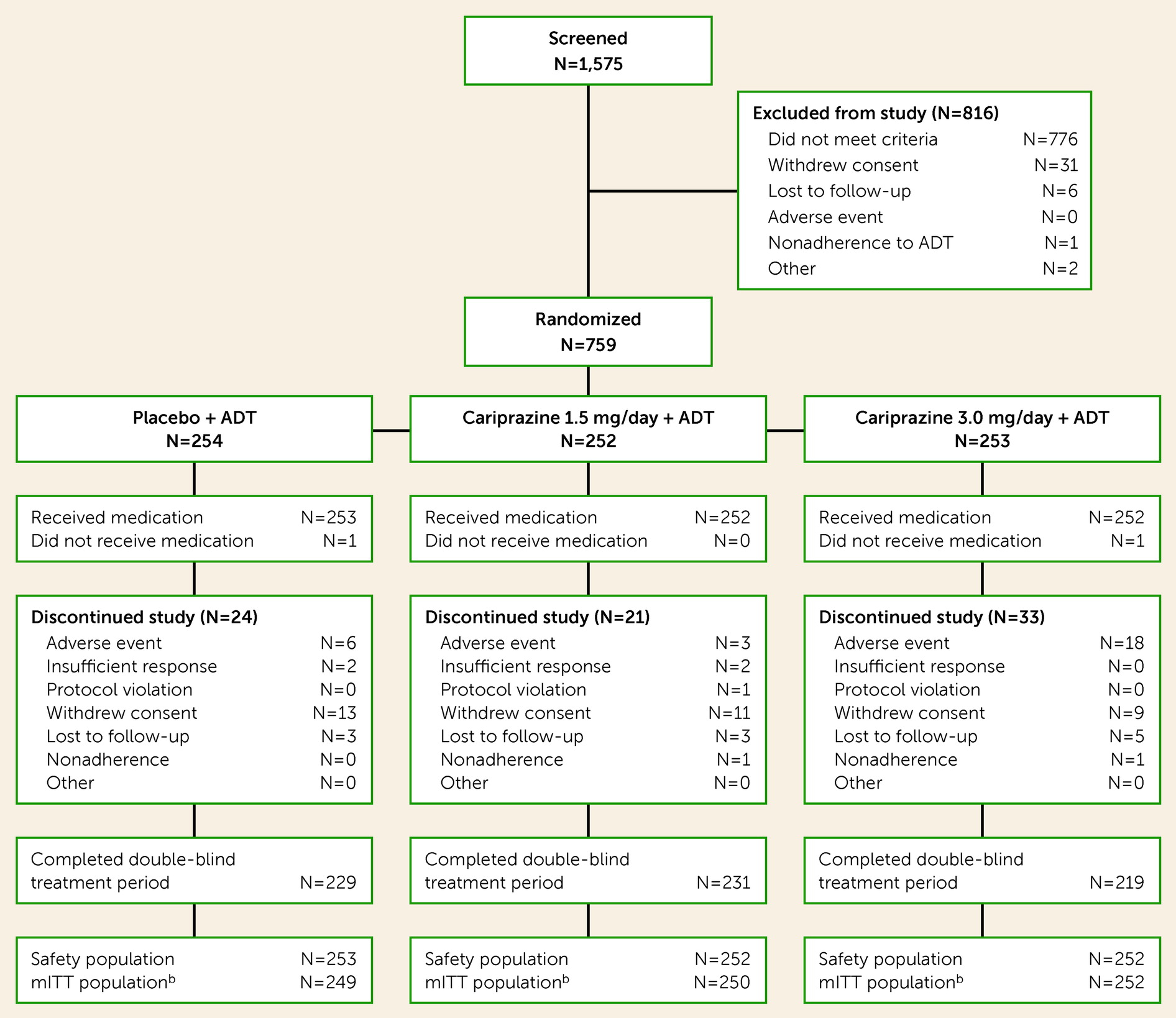
Of 1,575 patients screened for eligibility, 759 were randomized to double-blind treatment, 757 were included in the safety population, and 751 were included in the mITT population (
Figure 1). Completion rates were similar across the placebo (90.5%), cariprazine 1.5 mg/day (91.7%), and cariprazine 3.0 mg/day (86.9%) groups. Most premature discontinuations were due to adverse events (3.6%), loss to follow-up (1.5%), and withdrawal of consent (4.4%). Baseline demographic characteristics, clinical history (
Table 1), and efficacy scores (
Table 2) were generally comparable across treatment groups. Participants were presently taking one of 21 different ongoing antidepressants for at least 6 weeks during the current major depressive episode; most patients escalated the dosage at least once (see Table S1 in the
online supplement).
Efficacy Outcomes
Primary, secondary, and additional efficacy outcomes.
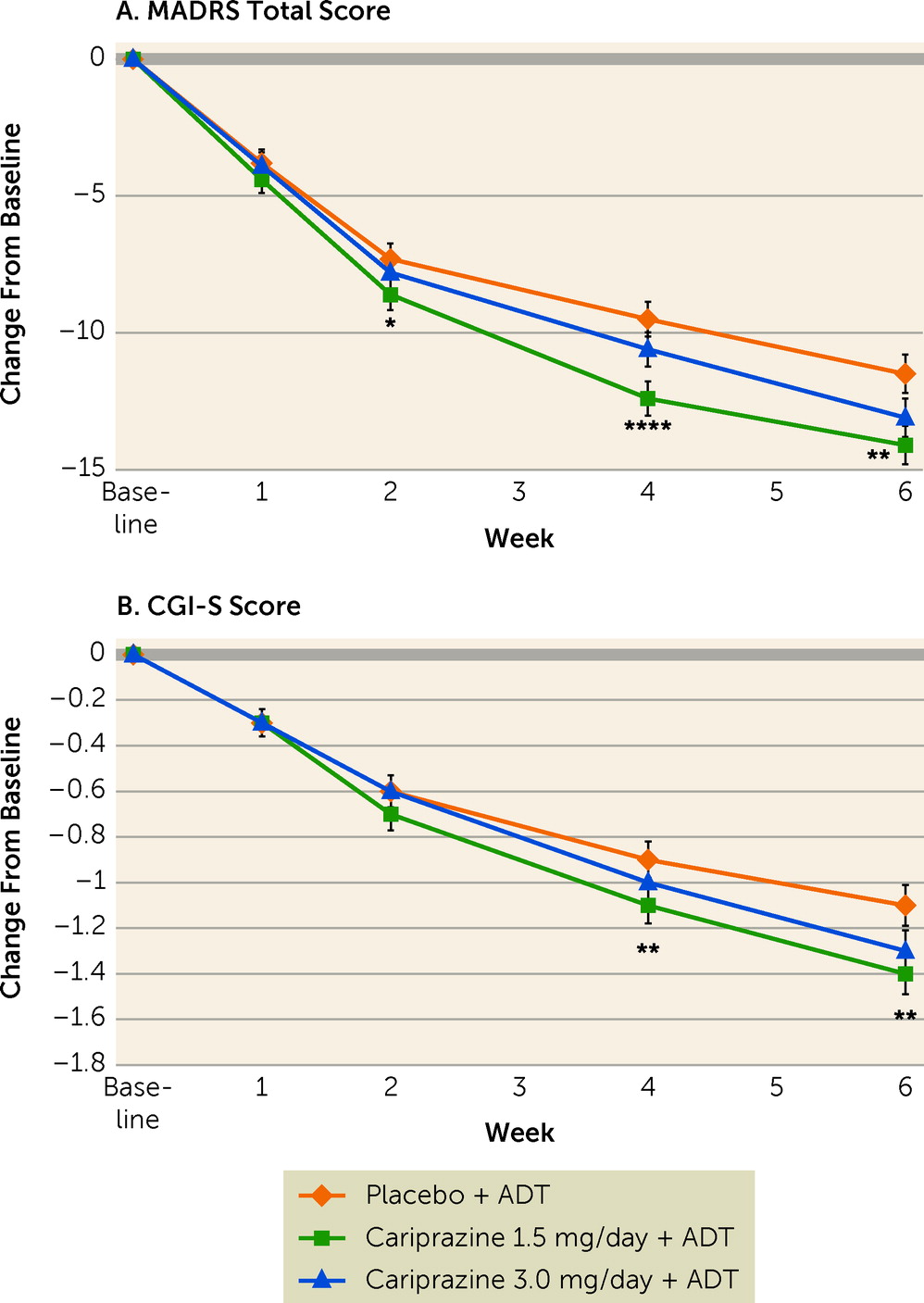
Mean MADRS total scores at week 6 were 19.5 (SD=10.3) in the placebo group, 17.4 (SD=9.1) in the cariprazine 1.5 mg/day group, and 18.6 (SD=8.9) in the cariprazine 3 mg/day group. Adjunctive cariprazine 1.5 mg/day compared with placebo resulted in significantly greater mean reductions in MADRS total score from baseline to week 6 (−14.1 vs. −11.5; p=0.0025; adjusted p=0.0050) (
Figure 2A;
Table 2); MADRS score reductions were significant for cariprazine 1.5 mg/day compared with placebo at week 2 (nominal p=0.0453) and week 4 (nominal p<0.0001). Cariprazine 3.0 mg/day compared with placebo resulted in numerically greater reductions in MADRS total score from baseline to week 6 (−13.1 vs. −11.5); however, this difference did not reach statistical significance (nominal p=0.0691; adjusted p=0.0727) (
Figure 2A;
Table 2). Results of the sensitivity analysis were consistent with the primary results.
The difference in change from baseline to week 6 in CGI-S score (secondary efficacy outcome) was significant for cariprazine 1.5 mg/day compared with placebo (nominal p=0.0091); significance was not retained after adjusting for multiplicity (adjusted p=0.0727) (
Figure 2B;
Table 2). Greater reductions in CGI-S score with cariprazine 1.5 mg/day compared with placebo were observed starting at week 4 (nominal p=0.0033) and maintained through week 6. Numerically greater CGI-S reductions were also seen with cariprazine 3.0 mg/day compared with placebo from week 4 through week 6, although these differences did not achieve statistical significance (
Figure 2B).
Response and remission outcomes are summarized in
Table 3. At week 6, rates of MADRS response were significantly greater with cariprazine 1.5 mg/day compared with placebo (44.0% vs. 34.9%; nominal p=0.0446), but not with cariprazine 3 mg/day (39.3%) compared with placebo (p=0.3409) (
Table 3). There were no significant differences in MADRS remission rates between placebo (23.3%) and either cariprazine dosage (1.5 mg/day: 25.2%, nominal p=0.3691; 3 mg/day: 16.7%, p=0.1155). At week 6, HAM-D total score reduction was significantly greater with cariprazine 1.5 mg/day than with placebo (−12.7 vs. −10.6; nominal p=0.0014), but not with cariprazine 3.0 mg/day (−11.9; p=0.0597). At week 6, the difference in HAM-A total score was significant in favor of cariprazine 1.5 mg/day compared with placebo (−9.1 vs. −7.8; nominal p=0.0370). Greater improvements as measured by CGI-I scores at week 6 were observed with both cariprazine 1.5 mg/day (nominal p=0.0026) and cariprazine 3.0 mg/day (nominal p=0.0076) compared with placebo; numerically higher CGI-I response rates were observed with cariprazine 1.5 mg/day (51.2%) and 3.0 mg/day (50.4%) compared with placebo (43.0%), but the differences did not achieve nominal significance (
Table 3).
Safety
Extent of exposure and treatment adherence.
Mean treatment duration for placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day was 40.5 days (SD=7.4), 40.4 days (SD=7.7), and 39.7 days (SD=8.6), respectively. Overall median treatment adherence (assessed by pill count at every double-blind study visit) was ≥99% in all treatment groups.
Adverse events.
The only treatment-emergent adverse events that occurred in ≥5% of patients in either cariprazine group and at twice the rate observed in the placebo group were akathisia and nausea (
Table 4). Overall discontinuation rates were 9.5%, 8.3%, and 13.1% in the placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day groups, respectively. The most common reasons for discontinuation were premature withdrawal from the study and adverse events (
Figure 1). Rates of discontinuation due to adverse events were 2.4%, 1.2%, and 7.1% in the placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day groups, respectively. The majority of treatment-emergent adverse events were mild or moderate in intensity (placebo group: 97.8%; cariprazine 1.5 mg/day group: 96.8%; cariprazine 3.0 mg/day group: 96.8%). During double-blind treatment, four serious adverse events occurred (placebo group: two [worsening of depression; multiple sclerosis]; cariprazine 1.5 mg/day group: one [“social stay” hospitalization]; and cariprazine 3.0 mg/day group: one [kidney infection]); no serious adverse event was considered treatment related.
Akathisia was the most common treatment-emergent adverse event related to extrapyramidal symptoms (placebo group: two [0.8%]; cariprazine 1.5 mg/day group: 13 [5.2%]; cariprazine 3.0 mg/day group: 20 [7.9%]). Restlessness was reported in four (1.6%), six (2.4%), and eight (3.2%) patients in the placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day groups, respectively. Rates of discontinuation due to akathisia (≤2%) and restlessness (<1%) were relatively low in each cariprazine group. Akathisia led to the premature discontinuation of five (2.0%) patients in the cariprazine 3.0 mg/day group and one (0.4%) patient in the cariprazine 1.5 mg/day group. All other adverse events related to extrapyramidal symptoms leading to discontinuation were reported in two or fewer patients in each cariprazine group. Excluding akathisia and restlessness, rates of treatment-emergent adverse events related to extrapyramidal symptoms were generally low for placebo (1.6%) and cariprazine (<5%). Treatment-emergent parkinsonism (Simpson-Angus Scale score ≤3 at baseline and >3 at any postbaseline visit) was observed in five (2.0%), two (0.8%), and seven (2.8%) patients in the placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day groups, respectively. Treatment-emergent akathisia (Barnes Akathisia Rating Scale score ≤2 at baseline and >2 at any postbaseline visit) was observed in 10 (4.0%), 25 (10.0%), and 35 (13.9%) patients in the placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day groups, respectively. More patients receiving cariprazine plus antidepressant treatment than patients receiving placebo plus antidepressant treatment used rescue medications for extrapyramidal symptoms or akathisia (cariprazine 1.5 mg/day group: three [1.2%]; cariprazine 3 mg/day group: eight [3.2%]; placebo group: one [0.4%]) and for agitation, restlessness, and hostility (cariprazine 1.5 mg/day group: two [0.8%]; cariprazine 3 mg/day group: five [2.0%]; placebo group: none [0%]).
Other safety parameters.
Changes in laboratory and other safety parameters were generally comparable among treatment groups, with no clinically relevant differences observed (see Table S2 in the
online supplement). Mean weight change at the end of double-blind treatment was less than 1 kg in all groups (placebo group: +0.11 kg; cariprazine 1.5 mg/day group: +0.68 kg; cariprazine 3.0 mg/day group: +0.78 kg). Body weight increases ≥7% occurred in two (0.8%) patients receiving placebo, 10 (4.0%) patients receiving cariprazine 1.5 mg/day, and three (1.2%) patients receiving cariprazine 3.0 mg/day (
Table 4). The proportion of patients with clinically significant treatment-emergent changes in cholesterol, glucose, and triglyceride levels were generally similar in the placebo and both cariprazine groups. No evidence of transaminase alteration that comports with Hy’s law (i.e., alanine aminotransferase or aspartate aminotransferase ≥3 times upper limit of normal [ULN] with concurrent total bilirubin ≥2 times ULN and alkaline phosphatase <2 ULN) was recorded. Suicidal ideation assessed with the Columbia-Suicide Severity Rating Scale occurred in 8.4%, 10.4%, and 6.7% of patients receiving placebo, cariprazine 1.5 mg/day, and cariprazine 3.0 mg/day, respectively; most events were in the least severe category (i.e., “wish to be dead”) (placebo group: 8.0%; cariprazine 1.5 mg/day group: 8.0%; cariprazine 3 mg/day group: 6.0%). There was no suicidal behavior in any treatment group. No treatment-emergent mania (YMRS score ≥16) was observed.
Discussion
Treating MDD is a clinical challenge because many patients do not respond to the initial antidepressant treatment (
3–
5), which potentially results in continuing disability due to depressive symptoms. In this phase 3 fixed-dose study in patients with MDD and an inadequate response to antidepressant treatment alone, cariprazine 1.5 mg/day plus antidepressant treatment effectively reduced depressive symptoms, as assessed by change from baseline to week 6 in MADRS total score. Improvements with cariprazine 1.5 mg/day compared with placebo were noted early in the course of treatment, with a significantly greater score reduction observed at week 2 that was maintained through week 6. Although a numerically greater mean reduction in depressive symptoms was seen with cariprazine 3.0 mg/day compared with placebo, the difference was not statistically significant. A numerically greater reduction in global disease severity (CGI-S score) was also seen with both cariprazine dosages, with nominal significance observed with cariprazine 1.5 mg/day compared with placebo. Cariprazine plus antidepressant treatment was generally well tolerated in patients with MDD.
In a previously reported phase 2 study (
22) evaluating flexible-dose cariprazine in adults with MDD and an inadequate response to ongoing antidepressant treatment, change from baseline to week 8 in MADRS total score was significantly greater with cariprazine at 2.0–4.5 mg/day compared with placebo (least-squares mean difference=−2.2; adjusted p=0.0114) but not with cariprazine at 1.0–2.0 mg/day (least-squares mean difference =−0.9; adjusted p=0.2404). The mean daily dose in the cariprazine 2.0–4.5 mg/day group was approximately 3.0 mg/day, which supports a potential treatment effect for cariprazine 3.0 mg/day in reducing depressive symptoms. Although unipolar and bipolar depression are distinct illnesses, three previously published bipolar I depression studies (
36–
38) showed positive results with cariprazine 1.5 mg/day; cariprazine 3.0 mg/day showed positive results in one study, and positive trends were observed in the other two studies. Collectively, these studies support the efficacy of adjunctive cariprazine in reducing depressive symptoms.
There are several common challenges (
39) in MDD clinical trials that should be considered when interpreting the results from the present study, particularly those in the cariprazine 3.0 mg/day group. Given that titration to the 3 mg/day dosage occurred at study day 15 and the time to steady state is delayed due to the long half-life of cariprazine, the ability to detect a treatment effect in this dosage arm may have been compromised by the relatively brief 6-week double-blind treatment period. It should also be noted, however, that statistical separation from placebo occurred at week 2 with the 1.5 mg/day dosage, but not with the 3 mg/day dosage, even though both groups were receiving 1.5 mg/day at this point in the study, which suggests that dose effects may have at least partially reflected random variations in estimating the magnitude of modest antidepressant effects. Furthermore, the probability of receiving placebo has been shown to influence outcomes in clinical trials of MDD. In a meta-analysis, studies with two active-treatment arms (and therefore a 33% chance of receiving placebo), as in the present study, have been shown to raise the placebo response on average by almost 10% compared with studies with a 50/50 chance of receiving placebo (
40). Hence, the 34.9% placebo response rate in our study may have contributed to the lack of statistically significant differences for cariprazine 3.0 mg/day compared with placebo on the primary outcome and MADRS response.
Although the response rate in the present study was significantly greater with cariprazine 1.5 mg/day than with placebo, differences in the rate of remission were not statistically significant with cariprazine 1.5 mg/day (25.2%) or 3 mg/day (16.7%) compared with placebo (23.3%). Similarly, no significant differences in remission rates compared with placebo were detected in the earlier positive phase 2 adjunctive cariprazine study (cariprazine 1–2 mg/day: 31.9%; cariprazine 2–4.5 mg/day: 32.1%; placebo: 29.9%) (
22). Detection of treatment effects for remission in acute studies can be challenging given issues such as short study duration, study designs that are underpowered to detect between-group differences in remission, and high placebo response. Evidence suggests that a substantial proportion of remission in MDD occurs after 6 weeks of treatment, and patients who have already failed to remit with antidepressant treatment have a lower chance of doing so with each successive treatment (
41). To put our results in context with brexpiprazole, another recently approved atypical antipsychotic for adjunctive use in MDD, differences in remission rates compared with placebo were also not significant in any of their short-term clinical trials; in a pooled analyses of these studies, significant differences compared with placebo were detected, although remission rates were relatively low (brexpiprazole 2–3 mg/day: 16.2%; placebo: 12.6%; p=0.023) (
42). Interestingly, clinically relevant treatment effects on the primary outcome in both positive cariprazine studies were observed for the effective dosages (least-squares mean difference compared with placebo in MADRS total score change from baseline: −2.5 in the present study and −2.2 in the phase 2 study), which further suggest that while response and remission are informative measures, they should be considered within a wider scope of outcome data and inherent limitations.
Safety outcomes were consistent with the established safety profile of cariprazine across approved indications. Nearly all treatment-emergent adverse events reported during the double-blind treatment period were considered by investigators to be mild or moderate in severity. Akathisia, a known adverse event of cariprazine and other atypical antipsychotics, was one of the most frequently reported adverse events in this study; however, discontinuation due to akathisia was low in all treatment groups, suggesting that it was well managed in most patients. Mean weight increases were relatively low (less than 1 kg) in both cariprazine groups, and rates of weight gain ≥7% were also low (<5%). This profile of weight changes is consistent with an open-label, long-term safety study (
43) of adjunctive cariprazine in adults with MDD and inadequate response to antidepressant treatment, which reported mean weight gain of 1.6 kg over 6 months. Mean changes in metabolic parameters and shifts into abnormal ranges were infrequent and not thought to be clinically relevant, which is important because individuals with MDD who are receiving treatment with an atypical antipsychotic have a high risk of diabetes, obesity, and cardiometabolic disorders (
44). It should also be noted that no treatment-emergent mania occurred in either cariprazine group, suggesting that depressive symptoms improved without causing mood destabilization or manic switching in patients with MDD.
Strengths of the study include a fixed-dose design and adjustments for multiple comparisons to control for overall type I error. However, these results should be interpreted within the context of the study’s limitations, including a short trial duration, which may not have accommodated all tolerability issues given the long half-life of cariprazine, and the lack of an active comparator. Although up to three antidepressant treatment failures in the current major depressive episode were allowed, most participants had only one failure, indicating that the results are not generalizable to patients with treatment-resistant depression, which is commonly defined as at least two medication failures. Patients in this study met strict criteria for inclusion and exclusion, which may limit generalizability to other patient populations. Adherence to ongoing antidepressant treatment was monitored by patient report and pill count, if possible, during the study; however, the plasma level of the ongoing antidepressant treatment was not determined at screening, so adherence to antidepressant treatment before the study could not be verified. Additional investigation of the 3 mg/day dosage of cariprazine in studies with a longer duration is warranted, as is study of patients who do not respond to the 1.5 mg/day dosage but have few adverse effects and may benefit from a dosage increase.
In summary, this study demonstrated that cariprazine at 1.5 mg/day significantly reduced depressive symptoms in adults with MDD and inadequate response to antidepressant treatment alone. Cariprazine at 1.5 mg/day and 3.0 mg/day were both associated with favorable tolerability profiles, low discontinuation rates, and metabolic changes and weight gain that were not clinically significant. These results suggest that adjunctive cariprazine at 1.5 mg/day is an effective treatment for depressive symptoms in adults with inadequate response to ongoing antidepressant treatment, helping to address an unmet need among patients with MDD.
Acknowledgments
AbbVie and the authors thank the patients, study sites, and investigators who participated in this clinical trial. Caroline Warren, Pharm.D., of Prescott Medical Communications Group, provided medical writing support that was funded by AbbVie.