Understanding the neurocognitive mechanisms of efficacy in nonpharmacological pain interventions is a high-priority objective for improving pain treatment (
1). Mindfulness-based interventions are a category of nonpharmacological intervention in which participants train in awareness and acceptance of mental experience, commonly implemented via 8-week structured group programs such as mindfulness-based stress reduction (MBSR) (
2). Despite growing popularity and demonstrated benefits for a range of pain-related conditions and outcomes, our understanding of the neural mechanisms underlying mindfulness interventions for pain remains limited (
3).
Available mechanistic evidence on mindfulness-related pain modulation comes predominantly from brief laboratory interventions and cross-sectional study of long-term meditation practitioners (
4). Together, these studies suggest that mindfulness training may be associated with alterations in sensory processing circuitry as well as cognitive-emotional regulatory networks (
4–
6). However, no such study has yet been conducted on a standardized, full-length, and widely used clinical intervention, such as MBSR.
To address this gap in the evidence, we conducted a neuroimaging-based mechanistic investigation of pain response within a randomized, actively controlled trial of MBSR. To maximize the clinical interpretability of our findings, we applied a recently developed and well-suited method for analyzing functional neuroimaging of pain: behaviorally validated neural signature responses (
7,
8). These neural signatures comprise multivoxel patterns of neural activity that have been optimized for sensitivity and specificity to pain experience using machine-learning techniques (
7–
9). This approach offers several potential advantages for clinical mechanistic research. Relationships between signature response and subjective pain outcomes have been empirically established, addressing the common problem of “reverse inference” (
10) in conventional neuroimaging analysis. Thus, pain-related neural signatures are well-suited to provide mechanistic insights that bridge neural and psychological levels of analysis. Reflecting their status as potential neural biomarkers for pain, each signature also provides a single unidimensional response that can be analyzed alongside other outcomes, such as laboratory assays or symptom scales, using established clinical trial methodology (
11). Finally, traditional mass-univariate analysis for neuroimaging typically involves dozens or thousands of parallel hypothesis tests, requiring stringent corrections to control false positives and complicating estimations of effect size. For neural signature–based analysis, only a single such test is required per signature, increasing statistical power without inflating false positive rates, and furthermore allowing for unbiased estimates of effect size (
11).
In this study, we examined the effects of mindfulness training on two distinct aspects of pain processing using a complementary pair of signatures: the neurologic pain signature (NPS) (
7) and the stimulus intensity independent pain signature–1 (SIIPS1) (
8). The NPS was trained and subsequently validated to track mainly stimulus-dependent aspects of pain, that is, the intensity of pain reports induced by variations in noxious stimulus intensity. It comprises brain regions that show the most consistent activation to painful stimuli, especially those directly receiving afferent pain signals from the body. The NPS is reliably activated by multiple types of pain while responding minimally or not at all to other salient, emotionally evocative stimuli or to cognitive modulators of pain, such as placebo treatment (
8,
9,
12). The SIIPS1, by contrast, is designed to track stimulus-independent aspects of pain, and specifically, variation in pain reports not accounted for by stimulus intensity or NPS response. The SIIPS1 is not constrained to neural regions directly associated with nociceptive activity, and thus it incorporates a broader range of cognitive and emotional modulatory circuits. Importantly, the SIIPS1 has shown sensitivity to psychological modulators of pain processing in previous studies, including expectancy cueing and changes in perceived control, and thus it tracks cognitive elaborative processes that modulate pain experience independent of the sensory stimulus itself (
8).
To experimentally study the effects of mindfulness training, we randomized healthy, meditation-naive participants to a standardized 8-week MBSR course, a matched active control intervention validated in previous research (health enhancement program [HEP]) (
13), or a waiting list condition. Because much existing evidence in this area has been derived from study of long-term mindfulness practitioners, and because the pain-related cognitive processing involved has been theorized to change with mindfulness practice experience, we also examined a cross-sectional comparison sample of North American long-term mindfulness practitioners. The inclusion of this sample, along with the use of a consistent study protocol and pain task paradigm, was to allow for side-by-side study of short-term (MBSR) and long-term training effects.
We predicted that mindfulness training would reduce pain response on both subjective report and neural measures, with effects specific to duration of training. We tested predictions from past research (
4) via competing hypotheses. If mindfulness training modulates direct sensory signals, then reductions should be observed primarily in NPS response. Alternatively, if mindfulness training influences more elaborative stages of cognitive processing (
5), then reductions should be observed primarily in SIIPS1 response. We predicted that effects of short-term mindfulness training should be observed in the MBSR but not the HEP or waiting list conditions, while nonspecific effects should be common to both MBSR and HEP but not the waiting list condition. With regard to practice experience, we hypothesized that modulation of sensory signals, as observed in reported pain intensity and NPS response, should occur primarily in the early stages of training, as a result of greater use of effortful attentional mechanisms that may produce a gating effect on incoming nociceptive signals. In contrast, long-term training has been proposed to rely on less effortful mechanisms and more on consolidated changes in cognitive appraisal and elaboration, rather than sensory experience of pain (
4). We therefore hypothesized that greater differences in long-term practitioners would be observed in more stimulus-independent measures of pain unpleasantness and SIIPS1 response. Lastly, among long-term practitioners, we predicted that pain modulation would be related to the extent of practice experience. Given that our previous analyses have shown differing effects of mindfulness training according to the training context (
14,
15), we also separately examined the effects of practice experience accumulated through day-to-day practice and through intensive meditation retreats (
16), with the prediction that intensive retreat practice should produce stronger effects than routine daily practice.
Methods
Participant Recruitment
Healthy individuals were recruited and enrolled by logistical study personnel. A total of 127 meditation-naive individuals were assigned to one of three groups: an 8-week MBSR course, an 8-week HEP course as an active control group, or a waiting list control group with no intervention. Assignment was performed by a logistical staff member using computerized random number generation. Study measures were collected by experimenters who remained blind to group assignment during data collection. In addition to the meditation-naive participants, 31 long-term meditators were recruited for cross-sectional comparison with nonmeditators.
Participants were screened for the following exclusion criteria: cardiovascular and neurological health issues; history of psychotropic medication use; or history of psychiatric diagnosis other than depression, or within the past 5 years. Additional exclusion criteria for the meditation-naive group included prior experience with meditation or mind-body techniques, physical limitations, or extensive engagement in physical exercise. Inclusion in the long-term meditator group required at least 3 years of formal meditation experience, including multiple intensive retreats and ongoing daily practice. Data were collected as part of a larger study on mindfulness interventions for emotions and well-being at the University of Madison–Wisconsin from November 2009 to March 2012 (
14,
15,
17). The enrolled sample size, with a target of 30 per group allowing for dropout, was chosen based on findings from previous studies of neural changes associated with MBSR to allow detection of medium-sized effects (Cohen’s d=0.5) with a power of 0.80 at an alpha level of 0.05. The study procedures were approved by the University of Madison–Wisconsin Health Sciences Institutional Review Board, and participants provided written informed consent. Further details on study recruitment are provided in Supplemental Methods in the
online supplement.
Interventions
The primary intervention, MBSR, is an 8-week course consisting of instruction and practice in cultivating continuous focused attention on the breath, bodily sensations, and mental content while in seated postures, walking, and performing yoga (
18,
19). The active comparison intervention, HEP, is a non-mindfulness-based course matched with MBSR on length, structure, and nonspecific therapeutic elements, including a supportive group atmosphere, expert instruction, and positive expectancy for benefit (
13). Further details on the interventions are provided in Supplemental Methods in the
online supplement.
Task Design
A total of 20 thermal stimuli lasting 12 seconds, including an 8-second plateau at peak temperature, were delivered to the inside of the left wrist (the full task details are provided in the
online supplement). Thermal stimulations were separated by a distractor task and intervals for cued anticipation, recovery, and subjective ratings of intensity and unpleasantness. Participants rated the intensity and unpleasantness of each thermal stimulus on a 0–20 scale (
20). An equal number of thermal stimuli were delivered for two conditions, painful heat and nonpainful warmth, in counterbalanced order. During painful heat trials, participants received stimulation at a temperature previously calibrated to correspond to a rating of 14/20, adjusted downward for tolerability at time of scan in increments of 1°C if needed (range, 42°C–49°C). During nonpainful warmth trials, participants received stimulation calibrated to be detectable but not painful (range, 36°C–43°C). Further details on task design are provided in Supplemental Methods in the
online supplement.
Data Acquisition
MRI imaging was conducted in a GE X750 3.0-T scanner with an eight-channel head coil. Respiration belt signals were recorded continuously during imaging runs using BIOPAC equipment and AcqKnowledge software. Further details on acquisition sequences and image processing are provided in Supplemental Methods in the
online supplement.
Neural Signatures
Both the NPS and SIIPS1 neural signatures were independently derived (
7,
8) and were applied to this novel data set without further refinement. Preprocessed MRI data were prepared for neural signature analysis by contrasting neural responses to painful hot versus nonpainful warm stimuli collapsed across all 20 thermal stimuli, yielding one contrast image per participant. Computation of per-participant NPS and SIIPS1 responses was performed using a publicly available analysis script without modifications (apply_mask.m, available in the CANlab Core toolbox on GitHub;
https://github.com/canlab). Processing for the two signatures differed only in which of the corresponding signature response maps was used in the script. Scaling of the signature response depends on both study-specific parameters (as with all blood-oxygen-level-dependent functional MRI studies) and specific properties of each signature and is thus reported in arbitrary units. (The NPS is available for noncommercial research use with a signed material transfer agreement from Dr. Wager. The SIIPS1 is freely available for download from
https://github.com/canlab/Neuroimaging_Pattern_Masks.) Further details on development of the signatures, previous validation, component brain regions, and availability to researchers are provided in Supplemental Methods in the
online supplement.
Statistical Analysis
Final statistical analysis was conducted using R, version 3.3.2 (
https://www.r-project.org). Intervention effects on pain response were modeled using analysis of covariance (ANCOVA), regressing pre- to postintervention change on preintervention values. The potential covariates age and gender were examined for associations with pain outcomes at baseline and were included in intervention models when such associations were present. Effects of between-group differences for long-term meditators and meditation-naive participants and log-scaled lifetime practice hours for long-term meditators were computed using ordinary least-squares regression. Age, gender, mean respiration rate, and pain tolerance (maximum tolerable thermode temperature as determined by the calibration procedure) were examined as potential confounders for significant effects of practice hours. For neural pain signature responses, standardized effects are presented. For analyses of pain ratings (intensity and unpleasantness), respiration rate, and pain tolerance, effects are presented on the scale of measurement. Validation of pain signatures was performed using receiver operating characteristic analysis and an area-under-the-curve metric (
21) in addition to raw accuracy, with binomial tests versus chance performance. Standardized effect sizes are reported using Cohen’s d. The threshold for statistical significance was set at a p value of 0.05 (two-tailed). For purposes of potential further investigation, hypothesis-consistent effects with p values above the significance threshold but below a threshold of 0.1 are noted as marginal and are discussed separately.
Results
Sample Characteristics
Baseline analyses included 115 meditation-naive participants and 30 long-term meditators for whom valid pain task data were available. The long-term meditator and baseline meditation-naive samples did not differ significantly in age, gender, education level, or socioeconomic status measured with the Hollingshead index (
22) (all p values >0.05) and had minimal psychiatric history (see
Table 1 and Supplemental Results in the
online supplement). Intervention analyses included 91 participants with valid pain task data at both sessions (MBSR group, N=28; HEP group, N=32; waiting list group, N=31). Analyses involving respiration measures were based on 74 meditation-naive participants (MBSR group, N=24, HEP group, N=27; waiting list group, N=23) and 25 long-term meditator participants for whom valid respiration data were available. (See Figure S1 in the
online supplement for the study CONSORT chart.)
Validation of Neural Signatures
Performance of the neural signatures was validated at the baseline session. Both signatures showed good performance in discriminating between painful heat and nonpainful warmth at the participant level (SIIPS1: accuracy=0.77; NPS: accuracy=0.77; both p<0.001 compared with chance) (see Supplemental Results in the
online supplement for additional details). Discrimination was similar or better for painful heat versus anticipation, recovery, and retrospective pain reporting periods (all p<0.001) (see Supplemental Results in the
online supplement for additional details). Both signatures were positively associated with thermode temperature (SIIPS1: r=0.18, p=0.028; NPS: r=0.36, p<0.001), with SIIPS1 response positively associated with subjective reports (intensity: r=0.19, p=0.026; unpleasantness: r=0.18, p=0.27), while NPS response was positively associated with thermode temperature after controlling for SIIPS1 response (r=0.34, p<0.001).
Baseline Characteristics
Across all participants, age was negatively associated with both NPS response (r=−0.26, p=0.001) and SIIPS1 response (r=−0.31, p<0.001), and was therefore included as a covariate in subsequent analyses of neural signatures. Age was not associated with subjective pain reports, and gender was not associated with either neural signature or subjective reports. Full details are presented in Table S1 in the
online supplement. Overall, findings for training effects on both pain signatures and subjective pain ratings were unchanged by the inclusion or omission of age, gender, and respiration rate as covariates, except as otherwise noted.
Short-Term Training
Neural signature response.
The MBSR group showed a decrease in NPS response relative to the HEP group (d=−0.43, p=0.050) and a decrease in NPS response from pre- to postintervention assessment (d=−0.43, p=0.023) (see
Table 2 and
Figure 1 for all intervention effects). The MBSR group also showed marginal decreases in NPS relative to the waiting list group (d=−0.36, p=0.096) and in SIIPS1 relative to both groups (HEP group: d=−0.37, p=0.089; waiting list group: d=−0.37, p=0.087). The HEP group also showed a marginal decrease in SIIPS1 response relative to the waiting list group (d=−0.37, p=0.087). No other between-group or within-group effects were observed for neural signatures.
Subjective report.
The MBSR group showed a marginal decrease relative to the waiting list group (d=−0.39, p=0.078) and within the group from pre- to postintervention assessment (d=−0.38, p=0.028) (see
Table 2 and
Figure 1). The HEP group showed a decrease in unpleasantness relative to the waiting list group (d=−0.44, p=0.043) and a decrease within the group in both reported intensity and unpleasantness (intensity: d=−0.38, p=0.046; unpleasantness: d=−0.55, p=0.007). There were no other between-group differences in subjective report from pre- to postintervention assessment. There were no differences between or within groups in pain tolerance (see Table S2 in the
online supplement).
Respiration.
Within the MBSR group, mean respiration rate showed a decrease of 0.61 breaths/minute from pre- to postintervention assessment (95% CI=−1.21, −0.01, t=−2.10, df=23, p=0.047). However, decreases in respiration rate within the MBSR group were not significantly correlated with decreased neural or subjective pain response (see Table S2 in the
online supplement). There were no other differences between or within groups in mean respiration rate from pre- to postintervention assessment during the pain task (see Table S2 in the
online supplement).
Long-Term Training
Neural signature response.
No mean differences were observed between the long-term meditator and meditation-naive samples in neural signature response (see
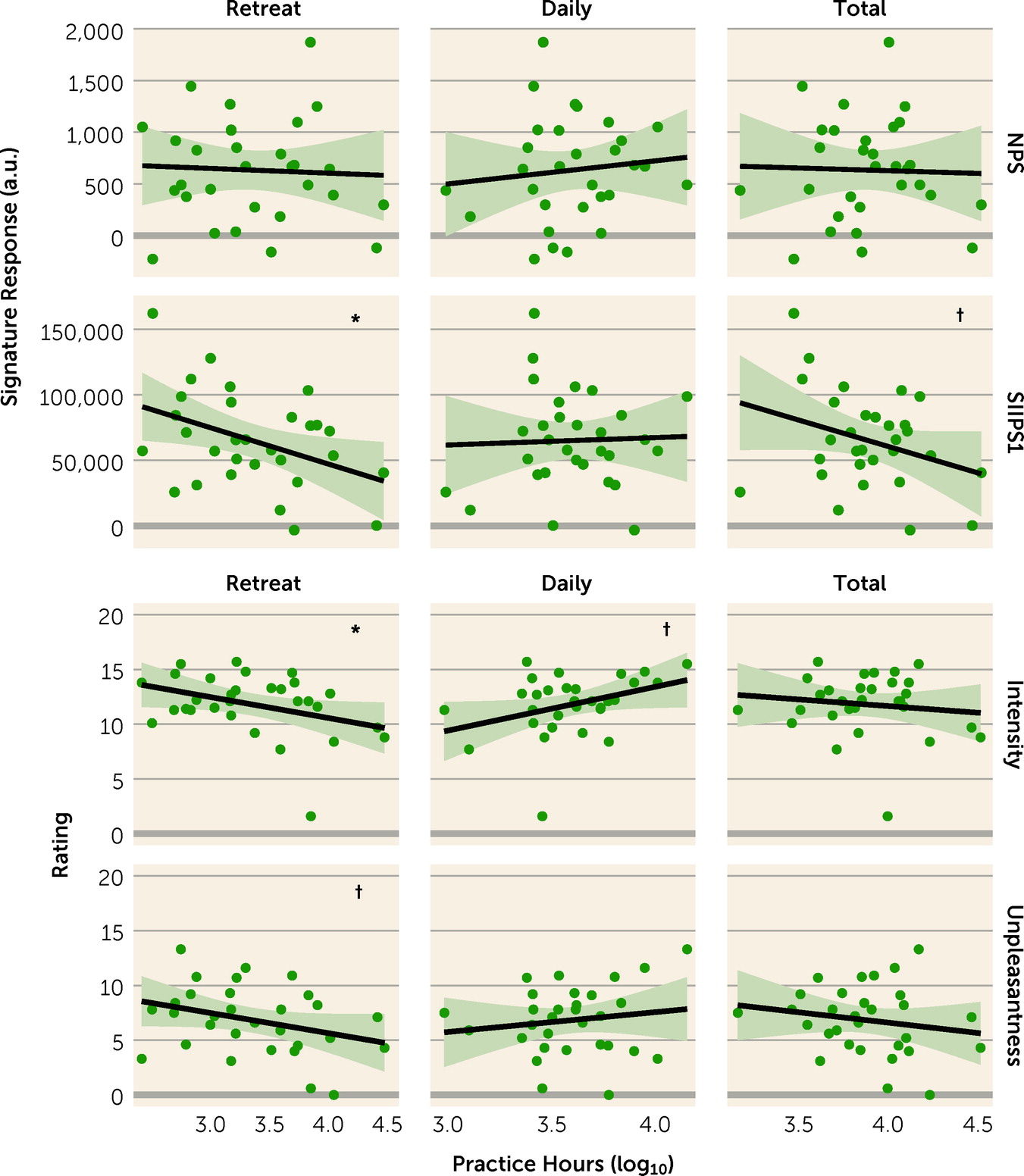
Table 3). Among long-term meditators, SIIPS1 response showed an inverse relationship with retreat hours (r=−0.65, p=0.027) (see
Table 3 and
Figure 2), which remained similar after further adjusting for gender and mean respiration rate (r=−0.45, p=0.031). No other relationships were observed between neural signature response and practice hours (see
Table 3).
Subjective report.
Long-term meditators reported less pain than meditation-naive participants (intensity: d=−0.54, p=0.001; unpleasantness: d=−1.05, p<0.001) (see
Table 3 and
Figure 2). Also, among long-term meditators, higher number of retreat practice hours was associated with reduced pain ratings (intensity: r=−0.37, p=0.046; unpleasantness: r=−0.32, p=0.085); these relationships remained similar after further adjusting for gender and respiration rate (intensity: r=−0.46, p=0.031; unpleasantness: 0.50, p=0.012) (see
Table 3 and
Figure 2). Daily practice hours showed a marginal association with increased pain intensity, contrary to our hypotheses (r=0.36, p=0.054); however, unlike findings for retreat practice, this was not robust to adjustment for gender and respiration rate (r=0.32, p=0.171). No other associations were observed between subjective pain reports and practice hours (see
Table 3). Pain tolerance did not differ between the long-term meditator and meditation-naive samples and was not associated with practice hours among long-term meditators (see Table S2 in the
online supplement).
Respiration.
Mean respiration rate during the pain task did not differ between the long-term meditator and meditation-naive samples and was not associated with practice hours among long-term meditators (see Table S2 in the
online supplement).
Discussion
We used neural pain signatures to identify the effects of mindfulness training on pain regulation. We confirmed in our sample that these signatures provided valid, objective measures of brain physiology related to pain and differentiated between two components of pain processing: direct, stimulus-related nociceptive activity and stimulus-independent elaborative cognition. Our first aim was to investigate the effects associated with short-term mindfulness training in the form of a standardized MBSR course. Our second aim was to look at practice-related differences in pain processing side-by-side for MBSR practitioners and a comparison sample of long-term meditators.
For MBSR training, as hypothesized, we observed a decrease in stimulus-dependent neural pain response (NPS) within the group as well as relative to the active control condition, and marginally relative to the waiting list condition. This decrease in neural response was paralleled by reduction in subjective pain unpleasantness, again both within the group from pre- to postintervention assessment and marginally relative to the waiting list group. In both the MBSR and HEP groups, only marginal decreases were observed for stimulus-independent response (SIIPS1) relative to the waiting list group. No within-group changes in SIIPS1 response were observed, leaving ambiguous to which group any such changes in response could be attributed. For long-term meditators, although subjective pain report differed from that of nonmeditators, neural response did not. However, among long-term meditators, greater retreat practice experience was associated with reduced neural pain response as well as reduced subjective pain; again, these changes were robust to the covariates examined. Overall, standardized effect sizes for short-term training fell in the small to medium range, while effect sizes associated with long-term training fell in the medium to large range.
Decreased response in the nociception signature (NPS) for MBSR aligns with earlier evidence suggesting that in early phases of training, mindfulness practice may have specific effects on stimulus-dependent sensory components of pain processing due to the common use of physical sensation as the basis for developing mindfulness skills. In particular, components of MBSR that emphasize body awareness, including body scanning practice and mindful movement, may play a role in this phenomenon. For the stimulus-independent pain processing response (SIIPS1), we observed potential reductions across both MBSR and the active control condition (HEP). Effects shared by both interventions could reflect nonspecific effects on pain response, which is known to be influenced by multiple social processes and expectancies found in in-person intervention settings (
23).
Although long-term meditators reported less subjective pain than nonmeditators, we did not find any group differences in neural response, whereas within the long-term meditator group, we did observe an association with cumulative practice. This somewhat surprising pattern is nevertheless consistent with previous findings on other measures; one potential explanation is that long-term commitment to meditation practice results in part from self-selection due to individual differences at baseline, which may then be remediated or reversed through extended practice. Among long-term meditators, we observed association with stimulus-independent pain response (SIIPS1), but not with stimulus-dependent response (NPS). This pattern was distinct from that seen in MBSR and suggests a possible shift in long-term mindfulness training, especially in intensive settings, from sensory-focused effects on pain toward more indirect, elaborative cognitive processes. Consistent with this pattern, evidence from previous studies of brief (1 week or less) interventions has suggested a transitional model whereby, initially, mindfulness training promotes modulation of both nociceptive sensation and elaborative, pain-related cognition, while in advanced training, sensory modulation decreases and cognitive modulation increasingly predominates (
4). Here, we extended these findings to the context of an MBSR intervention and provided further support for this model through side-by-side comparison of novice and experienced meditators using a common study design and a consistent experimental paradigm. Finally, we note that in long-term training, differences in neural pain response were associated with intensive retreat practice but not routine daily meditation. We have previously reported on distinct relationships between daily and retreat practice and other psychological and neurophysiological outcomes in long-term meditators (
14,
15,
24). One possible explanation for this finding is that on an hour-by-hour basis, intensive retreat practice, by facilitating longer sustained periods of practice and more intensive instruction, may support better consolidation of pain-relevant changes in cognition than shorter daily practice intervals (
3).
Alongside neural responses, we examined relationships between mindfulness training and subjective pain report, as well as considering the potential role of respiratory physiology in these findings. Although the effects we observed were modest, MBSR and long-term retreat practice were both associated with reductions in pain unpleasantness, as was the active control intervention. Long-term meditators also reported lower pain intensity relative to nonmeditators. Notably, for subjective reports, while we observed potential reductions for both interventions, we did not observe differentiation between MBSR and the active control condition. Thus, the MBSR-specific reduction in neural signature response may reveal an intervention-specific and psychologically interpretable change in pain response that is not accessible solely through subjective report. Finally, across conditions, respiration rate did not account for differences in pain response related to mindfulness training, reinforcing evidence that such differences are subserved by cognitive and affective processes and not solely physiological ones.
Our study had several limitations. For practical reasons, analyses of long-term mindfulness training relied on a cross-sectional practitioner sample and are thus associative in nature. Regarding clinical applicability, the study was conducted in a healthy community population, which may play a role in the modest size of observed effects. The observations reported here provide foundational estimates and ranges for the effects studied; however, as in any mechanistic clinical research, precise pinpointing requires cumulative evidence from multiple studies. Finally, our experimental approach relied on an acute pain paradigm optimized for compatibility with neuroimaging. The SIIPS1 signature incorporates activation in multiple regions associated with the transition from acute to chronic pain (
25); however, as yet, neural signatures specific to chronic pain have not been developed. Thus, further study will be needed to confirm how these findings apply in the specific contexts of chronic pain, pain-related medical conditions, and the presence of other psychiatric comorbidities.
Better understanding of nonpharmacological interventions for pain is an urgent challenge for clinical neuroscience. Furthermore, because pain is a complex biopsychological phenomenon, research is needed that integrates validated measures at both neurological and psychological levels to study individual aspects of pain. Here, we applied this approach in a randomized, actively controlled study design to provide the first neuroimaging study of changes in pain processing associated with a standardized mindfulness intervention in wide clinical use. Behaviorally validated neural pain signatures provided novel evidence for mechanisms of pain modulation in MBSR and differences in pain modulation between short-term and long-term mindfulness training. These findings advance our understanding of pain regulation mechanisms associated with nonpharmacological interventions. They also expose specific targets that can be leveraged in future research to optimize the efficacy of mindfulness-based interventions for pain.
Acknowledgments
The authors thank Michael Anderle, Ron Fisher, Lisa Angelos, Heather Hessenthaler, Trina Nelson, Amelia Cayo, Michael Kruepke, Jon Brumbaugh, Sarah Christens, and Andrew Schoen for assistance with data collection and Diane E. Stodola, John Ollinger, Rasmus Birn, John Koger, and Nate Vack for technical assistance. The authors also express their appreciation to the teachers of the MBSR and HEP protocols.