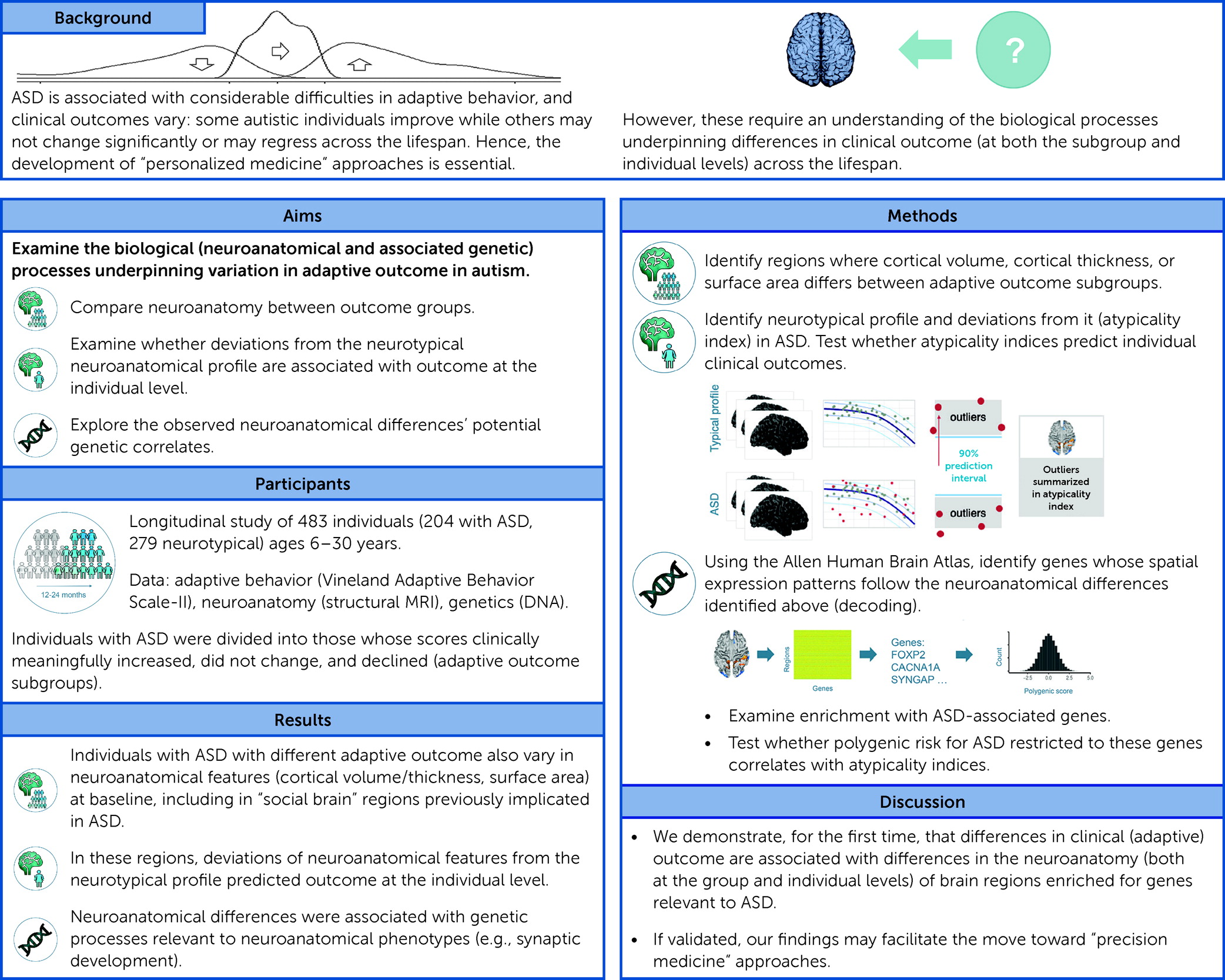
Currently, the best predictor of poor adaptive outcome in ASD is low overall cognitive functioning (IQ) (
10,
11). However, a significant proportion of autistic individuals with average or above-average IQ also have poor long-term social outcomes (e.g.,
12). Hence, we need to identify additional drivers of adaptive outcome, such as brain function and neuroanatomy. There is preliminary evidence that variation in brain function—that is, resting-state functional connectivity in specific networks—may predict adaptive outcome in ASD (
13). That finding constitutes an important first step, but the study had a moderate sample size (N=31), only included male adolescents and young adults without intellectual disability, and focused on brain function at a group level. Moreover, several studies have reported a link between neuroanatomy (e.g., amygdala volume [
14], local gray matter volume [
15], and white matter integrity [
16]) in children with ASD and cross-sectional measures of adaptive behavior. Further, we and others recently linked variability in neuroanatomical features to variation in genes that are mechanistically relevant to these features (
17,
18). However, no study to date has examined the neuroanatomical (and associated genetic) correlates of change in adaptive behavior at both the subgroup and individual levels across age.
Discussion
We examined, for the first time, the neuroanatomical correlates of change in adaptive behavior in ASD over a period of ∼1–2 years at both the group and the individual level. We demonstrate that ASD subgroups with different adaptive outcomes at follow-up were distinguished by widespread neuroanatomical differences at baseline. Moreover, in ASD, at an individual level, absolute deviation from the neurotypical neuroanatomical developmental profile predicted subsequent adaptive outcome. In regions that differed neuroanatomically between ASD subgroups, we discovered 1) enrichment for genes known to be transcriptionally downregulated in cortical tissue in ASD, and 2) an association between deviations from the typical developmental trajectory and polygenic variation (in the genes expressed here) associated with ASD. Notably, in predicting adaptive outcome, neuroanatomical profiles carried weights similar to IQ. Also, subgroups differed neurobiologically but not in IQ. Hence, analyzing phenotypic features other than IQ (i.e., brain anatomy) may help us better understand putative mechanisms underpinning variation in adaptive outcome. Taken together, our study findings are among the first to characterize neuroanatomical (and associated genetic) anomalies associated with adaptive outcome in ASD.
Before discussing our findings and their implications, we will highlight several methodological considerations.
Our study sample covered a broad age range (6–30 years). Taking such a truly dimensional approach without applying arbitrary age barriers allowed us to examine real-life heterogeneity across the autism spectrum. Nonetheless, we recognize that the processes underpinning adaptive behavioral change may vary across developmental stages and follow-up durations. For instance, a 6-year-old may be expected to develop differently over 2 years compared to a 30-year-old. Therefore, we also repeated our analyses covarying for age, follow-up duration, and their interaction. To further preclude confounding through age, we repeated our analyses in a subset of participants matched for age. Last, to disentangle the processes underpinning adaptive outcome across age, and also to make our findings more relevant to previous studies examining individual age groups (e.g., children or adults), we repeated our analyses separately in children/adolescents and adults. Taken together, these analyses revealed associations between neuroanatomy and adaptive outcome in ASD across the lifespan, and perhaps especially in children/adolescents.
Further, our analyses were centered on the VABS-II, a single instrument to assess adaptive behavior. This decision was based on the fact that the VABS-II remains one of the very few measures in ASD with an evidence-based MCID estimate, that is, for which there exists an empirical measure of what constitutes a clinically meaningful change over time (
22). Further, the VABS-II measures communication/socialization skill and therefore captures ASD core deficits. This utility of the VABS-II is reflected in its use as a primary endpoint in registrational phase 3 trials in ASD (e.g., NCT03504917). Also, the focus of this work on adaptive behavior follows the prespecified analytic plan of the EU-AIMS project (described in reference
9). Nonetheless, we extended our analyses to also examine the association between neuroanatomy and change in behavior based on alternative measures of social communication symptoms, including the ADOS total and social affect score, and the SRS. Combined, these analyses revealed an association between neuroanatomy and behavioral outcome in ASD regardless of the type of communication/socialization measure. However, and unsurprisingly, neuroanatomical spatial patterns varied across symptom measures.
We were not yet able to replicate our findings by, for example, using other publicly available ASD data sets. This is because other samples do not possess the same breadth of phenotyping, sample heterogeneity, and longitudinal approach from childhood to adulthood as LEAP does (
21). We will address this limitation once suitable data sets become available. Also, we have not yet validated our findings and explored their potential in clinical settings. We plan to address this in future work. First, we aim to validate our findings, especially at the case level, using multivariate pattern analysis and machine learning approaches. Second, we will test the utility of our neuroanatomical findings as stratification markers to enrich trials, for example, for individuals likely to have relatively poor outcomes. Third, we will investigate the contexts of use (e.g., in children/adolescents vs. adults) in which our findings may best inform clinical trials.
In view of these considerations, we first identified spatial patterns of neuroanatomical variability associated with change in adaptive behavior at the group level. Existing studies have primarily examined the relationship between adaptive behavior and neuroanatomy cross-sectionally and within well-defined developmental stages (
14,
15). Our work extends these analyses by using an accelerated longitudinal study design in a larger sample with a broader age range (including adults). We established that subgroups differed significantly at T1 across several morphometric features (cortical volume, surface area, and cortical thickness), including in frontal, temporal, parietal, and occipital areas. The anatomical differences included regions previously implicated in ASD, for example, the prefrontal cortex, temporal lobe, and precuneus (
37). They also comprised regions linked to social communication processing, attention, cognitive control, and motor learning in ASD, such as the parietal cortex, supramarginal gyrus (
38,
39), insula (
40,
41), lateral occipital cortex (
42), and lingual gyrus (
43). Also, these results were robust and survived correction for multiple (potentially confounding) methodological, brain, clinical, behavioral, and demographic factors, such as site effects, total brain measures, medication, IQ, age, follow-up duration and its interaction with age, or subgroup differences in age, sex, and IQ. Given our whole-brain approach, correction for multiple comparisons, and exploration of potential confounders, a clustering of differences in social brain regions adds plausibility to the suggestion that they play a key modulatory role in adaptive outcome (including social communication difficulties). Nonetheless, the extension of spatial patterns into regions associated with other cognitive/behavioral functions implicated in ASD (e.g., attention [
41]) suggests that mechanisms beyond social communication processing may also contribute to changes in adaptive behavior.
Taken together, our findings indicate an association between adaptive behavior and brain anatomical differences—but these are likely widespread (i.e., not restricted to one particular region). This association was particularly noticeable in children/adolescents, where supplementary analyses revealed greater between-group differences compared to the analyses in adults. These findings are unsurprising given strong previous evidence that the young brain is highly dynamic and plastic but becomes increasingly mature with age (
44). Notably, we also identified widespread neuroanatomical differences between outcome groups derived using alternative communication/socialization measures (ADOS total, ADOS social affect domain, and SRS), further corroborating the link between neuroanatomy and variation in subsequent behavioral change. However, correlations between our VABS-II-based and these supplementary findings were low to moderate. This is unsurprising given the difference in the included participants and the fact that the VABS-II, while capturing the social communication domain, is an independent instrument measuring different and/or additional behavioral features compared to the ADOS/SRS (e.g., daily living skills). Notably, this inconsistency between findings derived through the most commonly used measures of social-communication difficulties in ASD further highlights the significant heterogeneity in ASD and the challenges inherent to characterizing the autistic brain. Combined, our findings suggest that at the subgroup level, change in adaptive behavior, especially in early life, but also in adulthood, is underpinned by regional variation in neuroanatomy at baseline. However, to better understand the developmental origins of adaptive outcome, it is important to examine how change in adaptive behavior relates to different morphometric features at the individual level, not just the group level.
Next, we therefore examined whether atypical neuroanatomy at T1 predicted subsequent adaptive outcome in ASD at the individual level. Previous studies have linked neuroanatomical variation to ASD symptoms, including adaptive behavior (
45,
46). We built on this work by demonstrating that where subgroups differed neuroanatomically at baseline, an individual’s deviation from the neurotypical profile predicted their subsequent adaptive outcome. Specifically, more abnormal frontal, temporal, parietal, and occipital cortical volume and frontal, temporal, and parietal surface area predicted a decline in adaptive behavior. Adding neuroanatomical predictors to our model (beyond age, IQ, etc.) increased the amount of explained variance in adaptive outcome.
Nonetheless, the implications of our findings remain to be explored. Atypicality indices provide a means to gauge “accumulated” neuroanatomical atypicality (similar to PGSs in genetics). However, as summary indices, they do not differentiate between positive and negative deviations from the neurotypical range or between individual regions. Similarly, they do not allow inferences about the biological mechanisms contributing to these atypicalities. For instance, it is unclear whether deviations above and below the estimated neurotypical range are driven by similar or different biological mechanisms. Also, our analyses do not allow inferences about whether or how the neuroanatomical atypicality quantified by atypicality indices contributes to subsequent clinical outcome. For example, it is unclear whether or how neuroanatomical atypicality in particular regions increases “susceptibility” to a specific adaptive outcome, and whether this “susceptibility” varies depending on which cortical feature is affected. Last, the implications of atypicality index–based findings may vary depending on the sample used to establish the neurotypical developmental trajectory. For instance, a broad age range (as used in our sample), may increase the generalizability but also diminish the temporal specificity and sensitivity of the derived atypicality indices. Taken together, our findings suggest that specific neurodevelopmental processes modulate change in adaptive behavior in ASD, but that it is the amount of (widespread) deviation from neurotypical neuroanatomy that predicts (poor) adaptive outcome—and not abnormalities in one specific brain region per se. Additional studies are needed to determine how neuroanatomical variation affects outcomes in other ASD symptom domains.
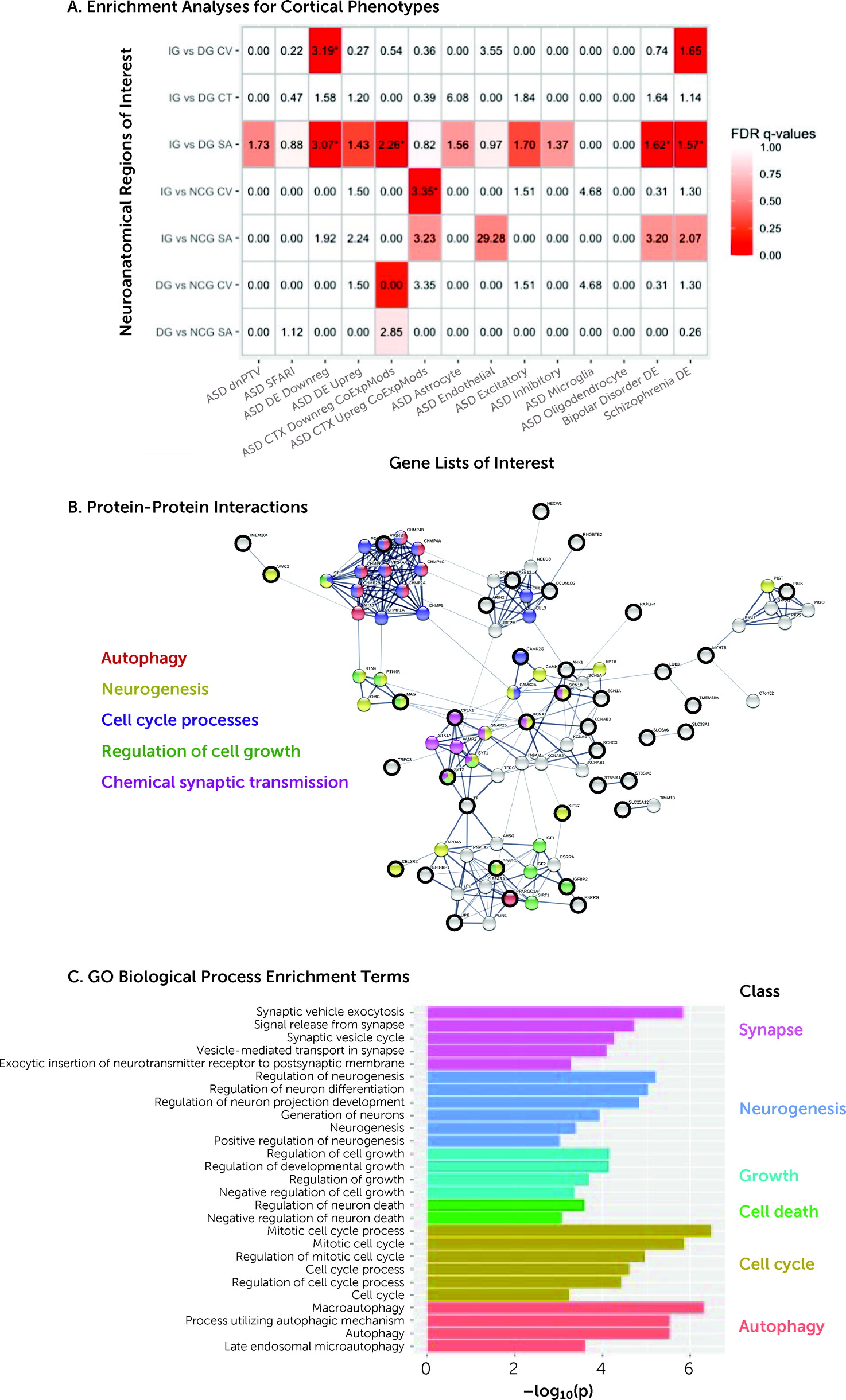
The mechanisms underpinning our group- and individual-level neuroanatomical findings are unclear. Previous studies suggest that cortical volume, surface area, and cortical thickness are regulated through different developmental processes (see, e.g.,
26,
27), which in turn are modulated through a complex interplay of genes (see, e.g.,
47–
49) and environmental factors. Accordingly, studies in rodents (
50) and humans (
17) have linked neuroanatomical variability to genetic variation. To build on this, here we examined the genetic correlates of outcome-related neuroanatomical variability. We observed enrichment for ASD-associated genomic mechanisms particularly in regions that differed between increasers and decreasers. Variability in cortical volume and surface area was associated with enrichment for genes transcriptionally downregulated in ASD postmortem cortical tissue. Previous studies have linked these genes (through GO enrichment analysis) to synaptic proteins, but this annotation may be incomplete. Specifically, many of these genes are also integrally involved in early processes associated with cell proliferation or neurogenesis (
51–
53)—that is, they have pleiotropic roles at different points in development that may affect mechanisms relevant to cortical volume and surface area and also the synapse. Moreover, neuroanatomical atypicality was correlated with genetic variation associated with ASD. Specifically, in regions differing between increasers and decreasers, greater deviation from the typical developmental trajectory of cortical thickness was correlated with a greater autism PGS restricted to the genes expressed here. This finding builds on previous reports associating (atypical) cortical thickness with genetic variation in autistic children (
17) by demonstrating a relationship between (atypical) neuroanatomy and genes not only globally but regionally, that is, in regions relevant to outcome, and not only in children but across age. Taken together, our findings suggest that particular aspects of adaptive outcome–related neuroanatomical variability in ASD may be accompanied by specific biological mechanisms relevant to these phenotypes.
In summary, we identified developmental differences in neuroanatomy and associated genetic factors that are linked to variation in subsequent adaptive behavioral change in ASD. If validated, our findings may enable the future stratification of patient groups, for example, to help target those more likely to have relatively poor outcomes, and enhance efforts to develop better targeted (personalized medicine) interventions in ASD.