Parkinson’s disease is the second most common neurodegenerative disorder after Alzheimer’s disease (
1), and its prevalence is expected to increase to more than 1 million patients in North America by 2030 (
2). Parkinson’s disease is commonly associated with psychosis (
3), and in patients with Parkinson’s disease, lifetime prevalence of visual hallucinations may approach 50% (
4).
Pimavanserin, a serotonin 5-HT
2 receptor antagonist, is the only drug approved in the United States for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. In premarketing trials of pimavanserin in patients with Parkinson’s disease psychosis, serious adverse events were more frequent during treatment with pimavanserin than with placebo (7.9% taking pimavanserin, compared with 3.5% taking the placebo in the 6-week trials), and during open-label pimavanserin treatment, 11% of patients died (
5). Postmarketing pharmacovigilance data on pimavanserin includes multiple reports of deaths (
6), but the data do not point to any specific, unexpected types of serious adverse events (
7).
A meta-analysis of placebo-controlled trials in patients with dementia-related psychosis that analyzed data on 3,353 patients treated with atypical antipsychotics and 1,757 treated with placebo found that the risk of mortality was roughly 50% higher with atypical antipsychotics (118 deaths [3.5%] compared with 40 [2.3%]) (
8). With respect to the Parkinson’s disease population, Parkinson’s disease psychosis has been treated off-label with atypical antipsychotics, which have dual dopamine and serotonin antagonism (
9). A prospective cohort study of 230 patients with Parkinson’s disease found that presence of psychotic symptoms was associated with roughly a 45% increase in mortality, although antipsychotic use itself was not a significant independent predictor of mortality (
10). However, a large retrospective cohort study of Veterans Health Administration patients with Parkinson’s disease (most without dementia) found higher mortality associated with antipsychotic use; as the authors noted, the comparison they made to antipsychotic nonusers may have been biased if the need for treatment with an antipsychotic was a risk factor for mortality (a situation known as confounding by indication) (
11). More recently, a retrospective cohort study of Medicare beneficiaries with Parkinson’s disease who were residing in nursing homes found that pimavanserin users had a 24% higher 30-day risk of hospitalization than nonusers. Also, mortality was higher for long-term pimavanserin users than nonusers; the adjusted hazard ratio for death at 1 year was 1.56 (95% CI=1.42, 1.72). However, as with the Veterans Health Administration study, the authors noted that the comparison between users and nonusers could be confounded by indication (
12). A single-center observational study of pimavanserin (N=113) and quetiapine (N=505) in Parkinson’s disease found, relative to untreated control subjects (N=784), an odds ratio for death of 1.74 (95% CI=1.15, 2.62) with quetiapine and 1.23 (95% CI=0.57, 2.68) with pimavanserin, adjusted for age and gender (
13). Another single-center retrospective cohort study included 92 patients with either Parkinson’s disease or Lewy body dementia who were treated with quetiapine or pimavanserin; death during follow-up occurred in seven (15%) of the quetiapine-treated patients and four (9%) of the pimavanserin-treated patients (p=0.88) (
14). Of note, mortality among patients with Parkinson’s disease using pimavanserin or atypical antipsychotics has not been compared in a large, nationally representative study.
To address knowledge gaps regarding mortality risks in patients with Parkinson’s disease treated with pimavanserin, we undertook a retrospective cohort study using U.S. Medicare data to evaluate all-cause mortality in patients with Parkinson’s disease using pimavanserin and atypical antipsychotics.
Methods
Data Source
Medicare is the U.S. government’s health insurance program for individuals age 65 years and older and for persons with end-stage renal disease or disability. We studied Medicare beneficiaries enrolled in fee-for-service Medicare Part A (hospitalization), Part B (outpatient medical care), and Part D (prescription drug coverage). We used anonymized Medicare administrative claims records to obtain data on inpatient or outpatient diagnoses and procedures, outpatient drug prescriptions, and deaths. As this retrospective analysis was considered a public health surveillance activity, ethics committee review and signed patient consent were not required by the U.S. Food and Drug Administration.
Study Population
We employed a new user retrospective cohort design to compare patients initiating treatment with pimavanserin or oral atypical antipsychotics. Eligible atypical antipsychotics were those available in the United States aside from clozapine, as it is available only through a special program because of the risk of severe neutropenia. From April 29, 2016, when pimavanserin was approved, through March 2019, patients were eligible for study inclusion if, on the date of qualifying prescription fill (index date), they had at least 1 year of continuous Medicare Parts A, B, and D enrollment, were age 65 or older, and during the year before their index date had at least one Parkinson’s disease diagnosis based on ICD-9 and ICD-10 coding (ICD-9 code 332, ICD-10 code G20), had at least one levodopa prescription, and had no prescriptions for pimavanserin or any antipsychotic. Patients were excluded if they received prescriptions for multiple atypical antipsychotics or received both pimavanserin and antipsychotics on the index date. Patients were also excluded if they were hospitalized on the index date or received hospice care in the prior year. Additionally, patients with a stay in a skilled nursing facility in the 30 days prior to the index date were excluded, as their prescriptions may not be reliably captured while in a skilled nursing facility. Patients with a chronic dialysis claim during the baseline period or who entered Medicare with end-stage renal disease were also excluded, as these patients may have greater mortality risk. We excluded patients with diagnoses of schizophrenia, schizoaffective disorder, or schizophreniform disorder during the baseline period, as these diagnoses may indicate a potential alternative indication for chronic atypical antipsychotic use.
Covariates
For the 1-year baseline period, Medicare claims data on chronic medical conditions, health care utilization, and dispensed medications were collected. We also used these data to produce values for the Charlson comorbidity index (
15) and the frailty index (
16,
17).
Follow-Up
Follow-up began the day after cohort entry and continued until whichever of the following came first: disenrollment from Medicare Parts A, B, or D; stopping treatment, defined by a gap in days’ supply between successive study drug prescriptions exceeding 14 days; dispensing of a nonstudy antipsychotic; switching from pimavanserin to an atypical antipsychotic or vice versa; end of the study period; or death.
Outcome
The primary outcome was death from any cause. Previously, we found high concordance between deaths identified in Medicare and the National Death Index (kappa=0.98) (
18). As a sensitivity analysis, we analyzed a composite outcome of death or admission to hospice.
Statistical Analysis
If users of pimavanserin differed in important characteristics from users of atypical antipsychotics, bias (confounding) could occur when comparing mortality risk. To address potential confounding, we used inverse probability of treatment weighting (IPTW) to estimate the average treatment effect in pimavanserin-treated patients (see the Supplementary Statistical Methods section in the
online supplement). We assessed balance on baseline characteristics (see Table S1 in the
online supplement) using standardized mean differences calculated before and after weighting, considering a standardized mean difference of ≤0.10 as a negligible imbalance in the baseline covariate (
19). Weighted Kaplan-Meier plots were used to characterize risk of death over time (
20). Weighted Cox proportional hazards regression with robust variance estimation was used to estimate the hazard ratio and 95% confidence interval for comparison of duration of survival among the atypical antipsychotic and pimavanserin groups. Our initial comparison revealed that the hazard ratio was not constant over the duration of follow-up, indicating that the proportional hazard assumption was violated. Consequently, we performed segmented proportional hazards modeling with time intervals determined primarily from inspection of Schoenfeld residual plots (
21). In addition, we calculated the cumulative number needed to harm for one additional death, based on time-to-event data (
22). To assess the potential for bias in the primary analysis, we calculated the E-value, which is the minimum strength of residual confounding required to fully explain away the observed treatment effect (
23).
In a secondary analysis, we limited our comparison to patients who received their index prescription for pimavanserin or atypical antipsychotics from neurologists, to enhance comparability and further reduce the possibility that atypical antipsychotic treatment was unrelated to Parkinson’s disease psychosis. Additionally, to assess whether the hazard ratio for mortality was different between beneficiaries who had been admitted to hospice and beneficiaries who had not, we estimated different hazard ratios during the interval before a beneficiary entered hospice and during the interval after a beneficiary was observed to have a hospice admission.
We conducted a priori subgroup analyses by age, gender, comorbid dementia (defined by a diagnosis or use of an antidementia drug during the year prior to index date), comorbid depression (defined by a diagnosis in the year prior to index date), nursing home residency on index date, Charlson comorbidity index, and frailty score. Post hoc, we analyzed dementia patients identified with antidementia drug use alone.
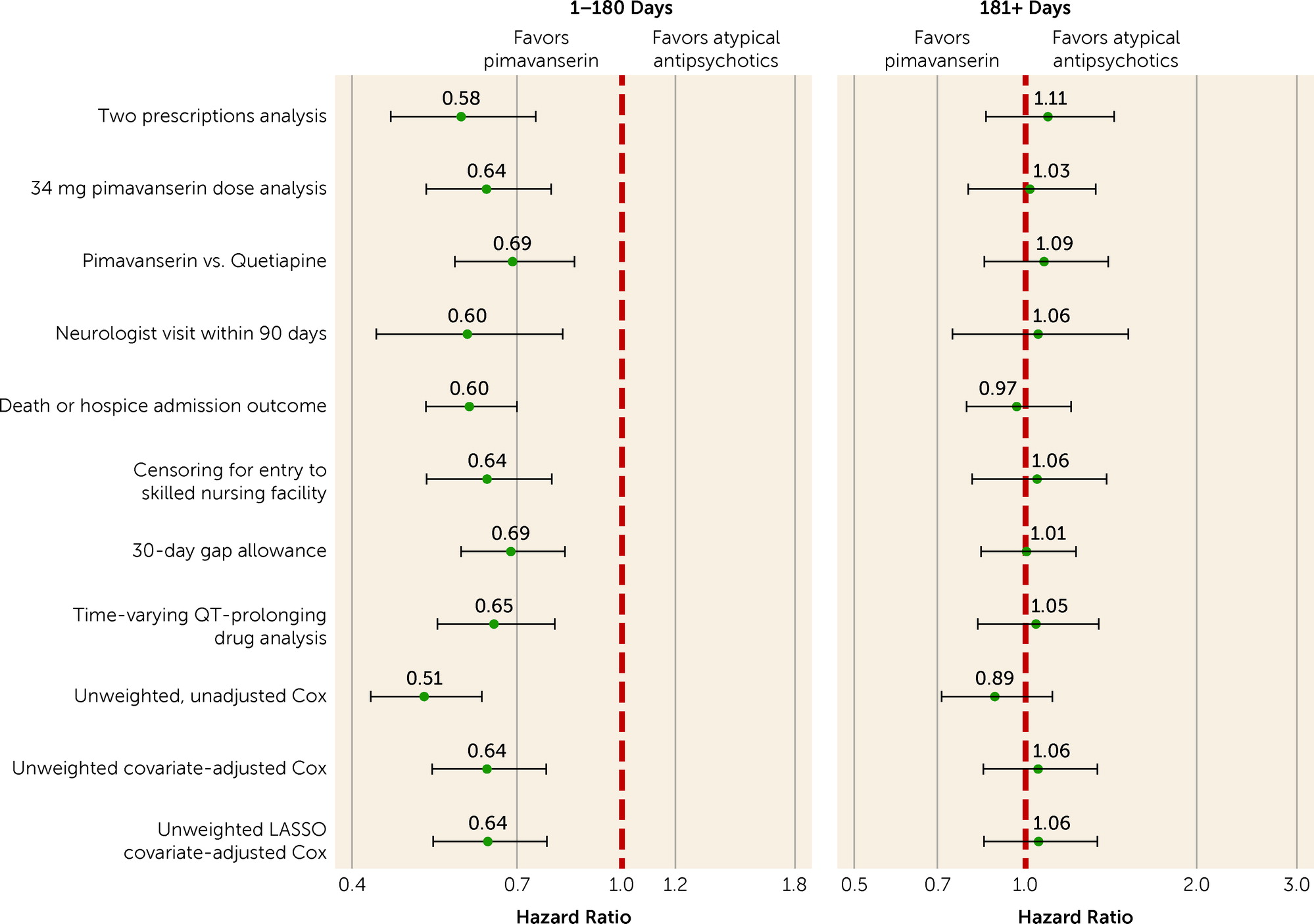
A priori sensitivity analyses included increasing the gap between prescription refills from 14 to 30 days; restricting the sample to patients with exposure defined by at least two prescriptions; restricting the sample to patients receiving exactly 34 mg/day of pimavanserin, the recommended daily dose; restricting the sample to patients receiving care from neurologists in the 90 days prior to the index date, to account for possible differences in management of Parkinson’s disease psychosis for these patients; and censoring for entry to a skilled nursing facility, to ensure capture of dispensed prescriptions. We also performed an analysis restricted to patients who received quetiapine as their initial atypical antipsychotic, and in a post hoc analysis, we compared pimavanserin users to users of non-quetiapine atypical antipsychotics. To assess whether there was a difference in risk of clinical deterioration preceding mortality, we also assessed a composite outcome of death or admission to hospice. Because pimavanserin prolongs the QT interval, it should be avoided by patients with congenital QT prolongation or other risk factors for QT prolongation, and it should not be combined with other QT-prolonging drugs (
24). Accordingly, we conducted an analysis accounting for concomitant use of potent QT-prolonging drugs (
25) (see Table S2 in the
online supplement) as a time-varying covariate. Finally, we repeated the analysis without any adjustment and without weighting, using multivariable Cox regression with all covariates, and covariates selected with LASSO regression (
26). We performed all secondary, subgroup, and sensitivity analyses using segmented Cox regression.
We used R, version 3.6.0 (R Foundation for Statistical Computing, Vienna) and SAS, version 9.4 (SAS Institute, Inc., Cary, N.C.) for statistical analysis.
Results
We identified 3,227 eligible pimavanserin-treated patients and 18,442 eligible atypical antipsychotic–treated patients (see Table S3 in the
online supplement). The median exposed follow-up time was 78 days (interquartile range=44–202) for pimavanserin and 74 days (interquartile range=44–170) for atypical antipsychotics. The reasons for censoring during follow-up were similar by cohort (see Table S4 in the
online supplement). Among atypical antipsychotic users, the most frequent initial atypical antipsychotic prescription was for quetiapine (in 78% of atypical antipsychotic users), followed by risperidone (9%), olanzapine (6%), aripiprazole (5%), and other atypical antipsychotics (1%). The mean age of pimavanserin-treated patients was 78 years, and 45% were female. Prior to weighting, compared to pimavanserin users, atypical antipsychotic users were more likely to visit a primary care provider and to have had a hospital admission in the 30 days before study entry. Certain comorbidities, including atrial fibrillation, chronic obstructive pulmonary disease, and diabetes, were more common in atypical antipsychotic users, and these patients’ mean Charlson comorbidity index and frailty scores were higher. Additionally, pimavanserin-treated patients tended to use higher doses and more classes of anti-Parkinson medications. After weighting, the cohorts were closely balanced on all covariates (
Table 1; see also Table S1 in the
online supplement).
During follow-up, 207 pimavanserin users and 1,752 atypical antipsychotic users died. Overall, pimavanserin users exhibited a higher cumulative survival probability through 360 days of follow-up (
Figure 1). The risk of death was lower among pimavanserin users (hazard ratio=0.77, 95% CI=0.66, 0.90). However, statistical evaluation showed that the proportional hazard assumption was violated. Guided by inspection of the Schoenfeld residuals (see Figure S1 in the
online supplement), we analyzed days 1–180 and days 181+ separately in a segmented Cox regression (
Figure 2). These segmented analyses satisfied the proportional hazard assumption and showed that mortality risk with pimavanserin was lower than with atypical antipsychotics during the first 180 days of use (hazard ratio=0.65, 95% CI=0.53, 0.79), while mortality risk was similar thereafter (hazard ratio=1.05, 95% CI=0.82, 1.33).
Table 2 enumerates the deaths during follow-up by time segment (1–180 days and 181+ days). During the first 180 days of follow-up, the adjusted death rates were 13 and 21 per 100 person-years with pimavanserin and atypical antipsychotic use, respectively. Corresponding rates of death after 180 days were 18 and 17 per 100 person-years for pimavanserin and atypical antipsychotics, respectively. At 75 days of follow-up, which was near the median duration of use for pimavanserin and atypical antipsychotics, 2.4% of pimavanserin initiators and 3.6% of atypical antipsychotic initiators had died, and at 180 days of follow-up, 3.7% of pimavanserin initiators and 5.4% of atypical antipsychotic initiators had died (see
Table 2 for weighted results). After 75 days, the number needed to harm for one excess death with atypical antipsychotic treatment versus pimavanserin treatment was 64 (95% CI=38, 220), and at 180 days of use, the number needed to harm was 30 (95% CI=19, 73). The secondary analysis limited to patients whose study drug was prescribed by a neurologist showed results similar to those of the main analysis (days 1–180: hazard ratio=0.61, 95% CI=0.47, 0.80; days 181+: hazard ratio=1.05, 95% CI=0.76–1.45).
Adjusting for hospice admission as a time-varying covariate eliminated the difference in mortality risk between pimavanserin and atypical antipsychotics (hazard ratio for death prior to hospice care, 0.91, 95% CI=0.72, 1.16; hazard ratio for death after hospice entry, 1.06, 95% CI=0.79, 1.42), consistent with hospice admission being on the pathway to the outcome of death. A total of 311 pimavanserin patients and 2,758 atypical antipsychotic patients died or were admitted to hospice care. The results for this composite outcome agreed with those for death alone, with a lower risk for pimavanserin users compared with atypical antipsychotic users in the first 180 days (hazard ratio=0.60, 95% CI=0.51, 0.70), but no difference in risk after 180 days (hazard ratio=0.97, 95% CI=0.79, 1.20).
A priori subgroup (
Figure 3) and sensitivity analyses (
Figure 4) were largely consistent with the main analyses, indicating lower mortality risk with pimavanserin use compared with atypical antipsychotic use during the first 180 days and little difference in mortality risk beyond 180 days. However, in the roughly 15% of patients residing in nursing homes, mortality was not lower with pimavanserin use compared with atypical antipsychotic use in the first 180 days (hazard ratio=1.05, 95% CI=0.73, 1.52) and in fact was numerically higher with pimavanserin in the 181+ days period (hazard ratio=1.54, 95% CI=0.91, 2.63) (
Figure 3). A statistical test for effect modification by nursing home residence was significant (p=0.008). In a post hoc analysis, we categorized patients by use of antidementia drugs in the baseline period. The difference in mortality between pimavanserin and atypical antipsychotic users was more pronounced in the subgroup treated with antidementia medications. In the first 180 days, hazard ratios for mortality with pimavanserin compared with atypical antipsychotics were 0.49 (95% CI=0.36, 0.67) and 0.82 (95% CI=0.63, 1.06) among users and nonusers of antidementia drugs, respectively. The hazard ratios for mortality during the 181+ days period were 0.92 (95% CI=0.66, 1.28) and 1.22 (95% CI=0.84, 1.75) for antidementia drug users and nonusers, respectively.
In one sensitivity analysis, we limited the atypical antipsychotic reference group to users of quetiapine, which was used by 78% of our atypical antipsychotic cohort (N=14,463). Daily quetiapine doses were generally low (79% were 50 mg or less). For pimavanserin versus quetiapine, over the first 180 days the weighted hazard ratio for death was 0.69 (95% CI=0.57, 0.85), and the hazard ratio for 180+ days was 1.09 (95% CI=0.85, 1.40). For pimavanserin versus non-quetiapine atypical antipsychotics, the hazard ratios for death were 0.54 (95% CI=0.37, 0.78) for the first 180 days and 1.24 (95% CI=0.75, 2.07) for 180+ days. The E-value for the observed hazard ratio during the first 180 days of treatment was 2.45, indicating that an unmeasured confounder would have to be more than twice as prevalent among patients using one medication as it was among patients using the other, and would have to alter the risk of death more than twofold, to explain the observed association.
Discussion
In a large, nationally representative population of Medicare beneficiaries with Parkinson’s disease, pimavanserin was associated with 35% lower all-cause mortality compared with atypical antipsychotics over the first 180 days of treatment, which translated to the avoidance of one death for every 30 patients treated with pimavanserin rather than atypical antipsychotics. The relative protective effect of pimavanserin was observed in 85% of the sample who were community-dwelling patients with Parkinson’s disease but not among the 15% of patients residing in nursing homes. Reductions in mortality with pimavanserin compared with atypical antipsychotics were more pronounced over the first 180 days among patients receiving antidementia medication. For the composite outcome of death or transfer to hospice care, there was a 41% lower risk for pimavanserin users compared with atypical antipsychotic users over the first 180 days of treatment.
Our data point to lower mortality with use of pimavanserin compared with atypical antipsychotics, but with two important exceptions. The first involves duration of use, as the relative mortality advantage for pimavanserin was absent beyond 180 days. Further research may be needed to clarify the reasons for that, as high attrition beyond 180 days was a limitation of our study.
The second exception to lower mortality with pimavanserin treatment was among nursing home patients, who comprised 15% of our study population. Mortality was not reduced with pimavanserin relative to atypical antipsychotics among these patients, which was a statistically significantly different finding than the result in community-dwelling patients. Furthermore, our data suggest that pimavanserin could be associated with higher mortality than use of atypical antipsychotics in nursing home patients with longer-term use. However, the relatively small sample size renders these estimates highly uncertain. Reasons for the discrepancy in results between nursing home and community patients are unclear. One possibility is that high baseline mortality among nursing home residents overwhelms the contributions of Parkinson’s disease psychosis treatments to mortality. Additionally, both initiation and continuation of medications for nursing home residents is subject to quality review under the Medicare program, while there is no corresponding third-party review of medication regimens for community patients, so these drugs may be used differently in the two settings.
As noted, a recent study of patients with Parkinson’s disease reported higher mortality among pimavanserin users than pimavanserin nonusers (
12). Our results do not conflict with this finding, however, because the reference group was nonusers; as psychotic symptoms in patients with Parkinson’s disease are associated with mortality (
10), pimavanserin users would be expected to have higher mortality than pimavanserin nonusers, three-quarters of whom in our sample did not use other antipsychotics.
Our study has several strengths. It was national in scope, which afforded us a large sample of pimavanserin users. Confounding by indication is an important limitation of studies involving comparisons with nonusers, who presumably have a lower prevalence of psychosis. We addressed this weakness by restricting our study to patients with Parkinson’s disease receiving treatment for probable Parkinson’s disease psychosis. We performed subgroup and sensitivity analyses to examine the potential for treatment-effect heterogeneity and to determine whether our results were robust to changes in the specifications of the analysis. Except for patients in nursing homes, our results were consistent across these analyses.
Several limitations of our study should be borne in mind. We were unable to compare mortality risk with pimavanserin to no medication, as we did not have a reliable way to identify patients with Parkinson’s disease with psychosis who were not receiving medication for it. Accordingly, our analyses only address the relative safety of pimavanserin in Parkinson’s disease compared to use of atypical antipsychotics. Importantly, because of the observational, nonrandomized nature of our study, our results may be subject to residual confounding, notwithstanding our analytic strategies to mitigate the effects of potential confounders. Before weighting, certain medical comorbidities were more frequent among atypical antipsychotic users at baseline. Weighting successfully ameliorated between-group disparities in all measured baseline covariates, including important predictors of mortality such as the Charlson comorbidity index and frailty score. The E-value estimate of 2.45 in the first 180 days of treatment suggests that unmeasured confounding was unlikely. Nonetheless, our study was not a randomized trial, and unmeasured confounding as an explanation for our results, although unlikely, cannot be excluded.
Heterogeneity in characteristics of pimavanserin users may have arisen over the study period if patients who received prescriptions for pimavanserin when it was newly marketed differed from those who initiated pimavanserin later. Heterogeneity may also have been present in indications for atypical antipsychotics, which have been used off-label for insomnia or agitation.
Additionally, we were unable to analyze specific causes of death. However, adjusting for hospice entry accounted for the difference in mortality risk, which would be consistent with a more gradual acceleration of the natural trajectory toward death among patients with Parkinson’s disease psychosis, rather than an acute event leading to increased mortality. Finally, with median follow-up times of 78 and 74 days for pimavanserin and atypical antipsychotic users, respectively, we had relatively fewer data on prolonged use of these drugs, although we still had reasonable statistical precision to analyze longer-term use.
In summary, our findings indicate a 35% lower rate of all-cause mortality among pimavanserin-treated patients with Parkinson’s disease in the first 180 days of treatment compared with patients treated with atypical antipsychotics. This effect was absent among patients residing in nursing homes.
Acknowledgments
The authors gratefully acknowledge Dr. Glenn Mannheim for proposing this study.