Safety of Psychotropic Medications in People With COVID-19: Evidence Review and Practical Recommendations
Abstract
Background:
Methods:
Results:
Conclusions:
Background
Methods
Results
| Review | Type of study | Population | Intervention | Comparison | Outcome | Summary | AMSTAR-2 |
|---|---|---|---|---|---|---|---|
| Clegg et al. 2011 (23) | Systematic review of observational studies and RCTs (no meta-analysis) | Patients from medicine and surgery settings, mostly elderly | Antidepressants Antipsychotics Benzodiazepines | No exposure or placebo | Risk of delirium | Increased risk for benzodiazepines as a class (1 case–control study; n = 1341; OR 3.0, 95% CI 1.3 to 6.8), with higher risk for longer-acting agents and higher doses; antipsychotics as a class (1 prospective cohort study; n = 325; OR 4.5; 95% CI 1.8 to 10.5), but not for haloperidol (1 RCT; n = 430; OR 0.9, 95% CI 0.6 to 1.3); and tricyclic antidepressants (1 RCT; n = 111; RR 1.7, 95% CI 1.4 to 2.1). | Critically low |
| Dragioti et al. 2019 (30) | Umbrella review (metaanalyses of observational studies) | Mixed (general population; people with depression) | Antidepressants | No exposure | Cardiovascular risk | Increased risk of coronary heart disease for TCAs (N = 14; n = 347,750; OR 1.51, 95% CI 1.07 to 2.12; CE IV). Risk of acute heart disease not increased for SSRIs (N = 14; n = 818,337; RR 1; 95% CI 0.83 to 1.22). | Critically low Critically low |
| Risk of myocardial infarction not increased for antidepressants as a class (N = 21; n = 1,793,877; RR 1.03, 95% CI 0.88 to 1.22). | Critically low | ||||||
| Increased risk of cerebrovascular disease for SSRIs (N = 6; n = 280, 784; RR 1.26, 95% CI 1.14 to 1.39; CE III). | Critically low | ||||||
| Risk of cerebrovascular disease not increased for TCAs (N = 4; n = 278,749; RR 1.06, 95% CI 0.96 to 1.17). | Critically low | ||||||
| Coagulation risk | Increase risk of severe bleeding at any site for SSRIs and SNRIs taken together (N = 44; n = 1, 443,029; OR 1.41, 95% CI 1.27–1.57; CE II). | Critically low | |||||
| Dzahini et al. 2018 (32) | Systematic review and meta-analysis of observational studies | Mixed (general population; people with schizophrenia, bipolar disorder, depression), mostly elderly | Antipsychotics | No exposure | Risk of infections | Risk of pneumonia is increased by FGAs (N = 5; n = 29,510, RR 1.69, 95% CI 1.34 to 2.15), SGAs (N = 6; n = 30,656; RR 1.93, 95% CI 1.55 to 2.41) and antipsychotics as a class (N = 7; n = 30,760; RR 1.83, 95% CI 1.60 to 2.10). No differences emerged between FGAs and SGAs. | Critically low |
| Huhn et al. 2019 (48) | Systematic review and network metaanalysis of RCTs | Adults with multiepisode schizophrenia | Antipsychotics | Placebo | Cardiovascular risk | Significantly increased risk of QTc prolongation (N = 51; n = 15,467): quetiapine (OR 3.43, 95% CI 0.94 to 6.0); olanzapine (OR 4.29, 95% CI 1.91 to 6.68); risperidone (OR 4.77, 95% CI 2.68 to 6.87); iloperidone (OR 6.93, 95% CI 4.49 to 9.36); ziprasidone (OR 9.7, 95% CI 7.43 to 12.04); amisulpride (OR 14.1, 95% CI 7.71 to 20.45); serenditole (OR 23.9, 95% CI 20.56 to 27.33). | Low |
| Kunutsor et al. 2018 (55) | Systematic review and meta-analysis of observational studies | General population | Antidepressants | No exposure | Coagulation risk | Increased risk of venous thromboembolism for | Critically low |
| antidepressants as a class (N = 6; n = 828,327; OR 1.27; 95% CI 1.06 to 1.51), TCAs (N = 4; n = 59,161; OR 1.16; 95% CI 1.06 to 27), SSRIs (N = 4; n = 58,088; OR 1.12; 95% CI 1.02 to 1.23), and other antidepressants (N = 3; n = 3198; OR 1.59, 95% CI1.21 to 2.09). | |||||||
| Lu et al. 2016 (59) | Systematic review and meta-analysis of RCTs | Adults with insomnia and COPD | Benzodiazepines | Placebo | Respiratory risk | No differences between benzodiazepines (i.e., triazolam and temazepam) and placebo in terms of percentage of time below 90% arterial oxygen saturation during sleep (N = 3; n = 94; weighted MD 1.32; 95% CI − 7.33 to 9.97) and other respiratory outcomes during sleep (sleep apnea, Apnea– Hypopnea Index, arterial oxygen saturation). | Critically low |
| Ostuzzi et al. 2019 (105) | Systematic review and meta-analysis of RCTs | Adults with depression and ischemic heart disease | Antidepressants | Placebo | Cardiovascular risk | No differences emerged for antidepressants as a class (SSRIs the most represented) in terms of mortality because of cardiovascular events (N = 14; n = 2674; RD 0.0, 95% CI − 0.01 to 0.01) and nonfatal cardiac events (N = 9; n = 1869; RD − 0.01, 95% CI − 0.04 to 0.02). | High |
| Papola et al. 2019 (70) | Umbrella review (metaanalysis of observational studies) | Mixed (general population; people with dementia, schizophrenia, or other psychiatric conditions), mostly elderly | Antipsychotics | No exposure | Cardiovascular risk | For antipsychotics as a class, there was an increased risk of sudden cardiac death (N = 6; n = 677,488; OR 2.24; 95% CI 1.45 to 3.46; CE III), myocardial infarction (N = 9; n = 399,868; OR 2.21; 95% CI 1.41 to 3.46; CE III), and stroke (N = 9; n = 65,700; OR 1.45, 95% CI 1.24 to 1.7; CE III). Meta-regression showed that the risk of myocardial infarction and stroke was higher in the elderly. | Moderate |
| Coagulation risk | For antipsychotics as a class, there was an increased risk of venous thromboembolism (N = 14; n = 31,417,175; OR 1.55, 95% CI 1.31 to 1.83; CE II). | Low | |||||
| Pollok et al. 2018 (72) | Systematic review and meta-analysis of RCTs | Adults with depression and COPD | Antidepressants | Placebo | Respiratory risk | No increased risk of respiratory impairment for TCAs (change in dyspnea during walk according to the Pulmonary Functional Status Instrument: N = 1; n = 30; MD 0.50; 95% CI − 1.34 to 2.34) and SSRIs (change in FEV1: N = 2; n = 148; MD 0.01; 95% CI − 0.03 to 0.05). | High |
| Schneider-Thoma et al. 2019 (78) | Systematic review and meta-analysis of RCTs | Mixed (83% adults; 8% elderly; 42% schizophrenia; 30% bipolar disorder; 11% depression; 6% dementia) | Antipsychotics (mostly second-generation) | Placebo | Respiratory risk | Increased risk of respiratory, thoracic, and mediastinal serious adverse events in studies with at least one serious adverse event according to a maximum estimate (worst-case scenario) | Low |
| (N = 38; n = 13,007; OR 1.72; 95% CI 1.02 to 2.89). | |||||||
| Cardiovascular risk | Risk of cardiac (N = 54; n = 19, 642; OR 1.22; 95% CI 0.85 to 1.75) and vascular serious adverse events (N = 33; n = 12, 842; OR 1.82; 95% CI 0.97 to 3.41) were not increased in studies with at least one serious adverse event according to a maximum estimate (worst-case scenario). | Low | |||||
| Risk of infections | Increased risk of infections in studies with at least one serious adverse event according to a maximum estimate (worst-case scenario) (N = 88; n = 28,479; OR 1.43; 95% CI 1.06 to 1.92). | Low | |||||
| Sun et al. 2018 (83) | Systematic review and meta-analysis of observational studies | General population, mostly elderly | Benzodiazepines | No exposure | Risk of infections | Increased risk of pneumonia for benzodiazepines and related medications (e.g., zolpidem) (N = 10; n = 1,520,285; OR 1.25; 95% CI 1.09 to 1.44). The risk was confirmed for current and recent exposure (not for past exposure); for long-acting, intermediateacting and short-acting agents; and for younger and older patients. | Critically low |
| Wu et al. 2019 (95) | Systematic review and network metaanalysis of RCTs | Medical and surgical patients at risk of delirium | Antipsychotics Benzodiazepines Mood stabilizers | Placebo/ treatment as usual | Risk of delirium | Decreased incidence of delirium as compared to placebo or treatment as usual (N = 38; n = 8168) emerged for olanzapine (OR 0.25; 95% CI 0.09 to 0.69) and risperidone (OR 0.27; 95% 0.07 to 0.99), while no differences emerged for lorazepam, haloperidol, and gabapentin. A higher incidence emerged for midazolam hydrochloride (OR 2.98; 95% CI 1.30 to 6.80). | Low |
Synthesis of the Evidence
Drug–drug interactions.
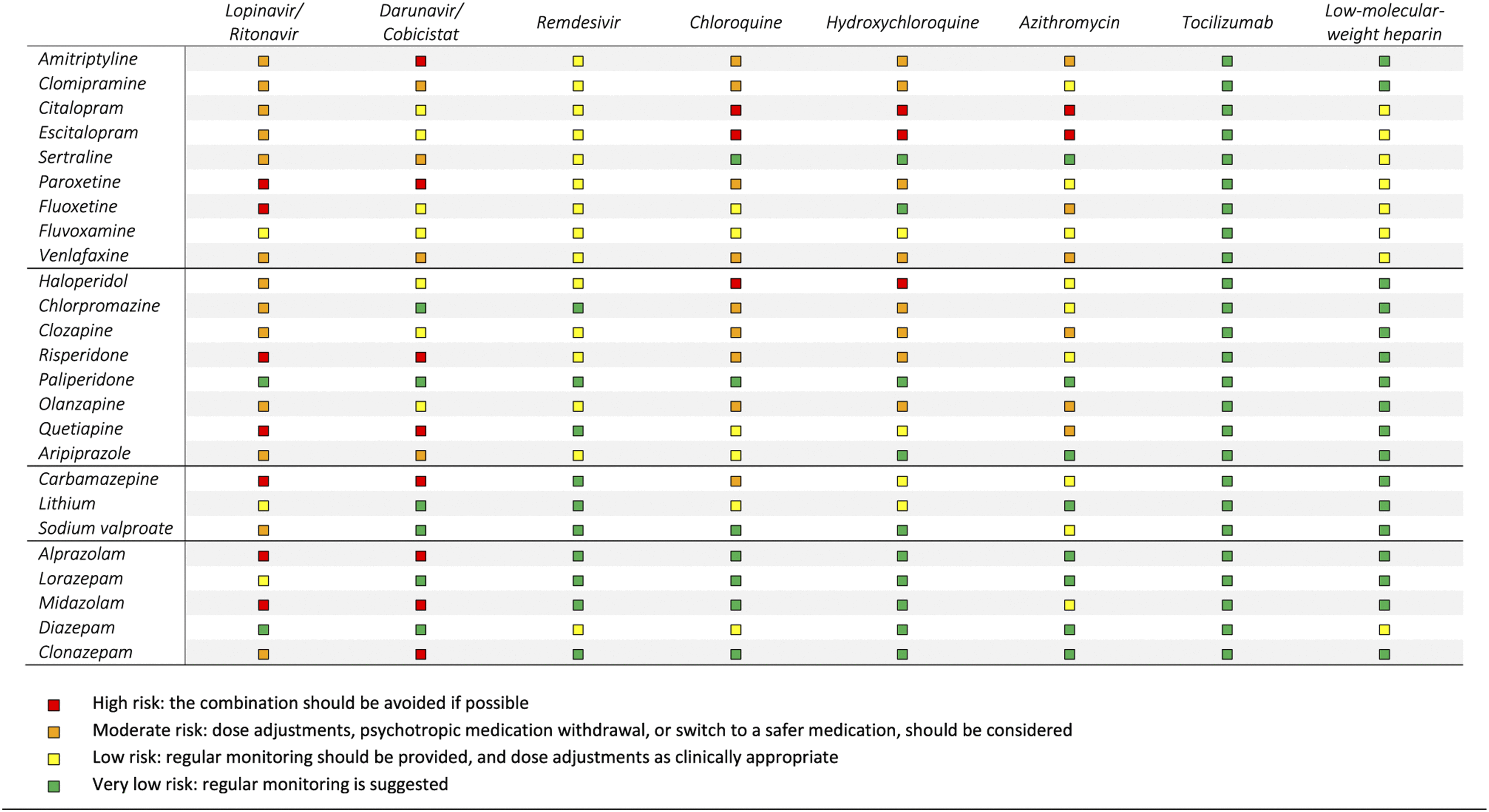
Respiratory risk.
Cardiovascular Risk.
Risk of infections.
Coagulation risk.
Risk of delirium.
Evidence-based Practical Recommendations
Discussion
Implications for Practice
Limitations
Conclusions
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

