Psychiatric disorders represent a public health concern, accounting for a significant portion of the general burden of disease—7.4% globally (
1). Unfortunately, of those treated, only few patients respond to first-line pharmacological treatments (
2). Also, both treatment resistance (
3) and treatment-limiting adverse effects (
4) are common, as well as high recurrence rates (
5). Further, psychiatric disorders often occur comorbidly with other medical conditions, posing additional challenges for pharmacological treatments (
6). Moreover, treatment challenges include overall access to providers and feasibility in treatment delivery, especially for psychotherapeutic options, and lack of treatment adherence (
7). Together, these challenges underscore the critical need to identify alternative treatment approaches.
The current unmet need for treatment has led to increasing development and evaluation of noninvasive brain stimulation (NIBS), expanding the body of knowledge concerning the role of neural circuits that underlie psychiatric disorders (
8), and highlighting the potential of NIBS as candidate interventions for these disorders (
9,
10). Specifically, once a target neural circuitry has been identified as a related region of interest for a psychiatric disorder, NIBS could be used to selectively modify activities in the target region (
11).
Among NIBS modalities, low-intensity transcranial electrical stimulation (tES) has been one of the most intensively investigated for both research and clinical applications, with particular promise for the treatment of psychiatric disorders (
12). The most common tES technique is transcranial direct current stimulation (tDCS), in which a constant, low-amplitude current is delivered through electrodes placed on the scalp to the regions of interest. Related techniques in development include transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS) (
13). A strong record of safety and tolerability has been established for the use of tDCS, associated with only mild and transient side effects (e.g., tingling and redness at site of electrode, mild skin erythema [
14]), and no serious adverse event has been reported across clinical trials to date. Skin lesions under the electrodes may occur but are rare and can be prevented by proper electrode preparation (
14,
15).
The potential uses of tES as a novel treatment approach for psychiatric disorders have been discussed elsewhere (
12,
17,
19,
20). Yet, because tES is an area of recent and rapid scientific and technological development, knowledge of and familiarity with its use in clinical settings remain limited among mental health professionals (
21). Thus, in this review, recent research examining the use of tES and its implications for the treatment of common psychiatric disorders in adults are discussed, primarily focusing on clinical findings of relevant controlled trials and meta-analyses (
Table 1) (
22–
26).
Basic Technical Aspects
A tES device is generally composed of the following items (
27): a microprocessor-controlled current source, a battery compartment with single-use batteries or a power outlet electrical cable for recharging the device, connection cables most commonly leading to an anode (red cable) and a cathode (blue cable) electrode, conductive rubber pads for the electrodes (which are sites of electrochemical reactions and should not be in direct contact with the skin), saline-saturated sponges to cover the rubber pads (alternatively, the sponges or rubber or silicon pads can be coated in gel or conductive cream), and nonconductive headgear, such as straps (
28), to fix the electrodes over desired cranial locations (
13) (
Figure 1). The number, size, and positions of the electrodes are called montage (
29). Typically, the term “electrode” refers to the rubber electrodes and the sponge together (
29).
The anode is the electrode where positive current enters the body, and the cathode is the electrode where positive current exits the body (
29,
30). In tDCS, the anode electrode and cathode electrode are fixed and defined, and these terms are used to describe the placement of each respective electrode. In tACS, because current regularly changes direction (with a timing defined by the stimulation frequency), the anode and cathode are not well defined—and so these terms are not used in tACS. In tRNS, the amplitude and direction fluctuate rapidly and irregularly (
29).
Nonfocal brain current flow is an attribute of a larger electrode surface area, which in the most traditional montages, ranges from 25 cm
2 to 35 cm
2 per electrode (
31), although the effects on the brain suggest electrode position-specific outcomes (
32,
33). The alternate technique of high-definition tDCS or tACS (HD-tDCS, HD-tACS, respectively) uses electrodes with a smaller surface area (<1 cm in diameter) arranged in an array. For example, the 4×1 ring array increases the focality of the brain current, allowing noninvasive targeting of cortical regions (
31,
34), whereas other HD-tDCS and HD-tACS configurations can target deeper regions simultaneously (
35).
Current intensity is measured in amperes, which quantifies the rate of electric charge that passes through a specific point every second. In conventional tDCS, where two electrodes are used, the direction of current in the anode is the opposite of the current in the cathode, and the intensity is the same as both electrodes. If multiple electrodes are used, such as in HD-tDCS, the intensity is calculated by the sum of the current in all anodes, in opposition to all cathodes (
14). Furthermore, electrode current density is calculated by dividing current intensity by the area of the electrode (e.g., 2 mA divided by 25 cm
2 yields an electrode current density of 0.08 mA/cm
2). The electrode montage, current, and duration of a tES session are the most important parameters that define stimulation dose (
13,
36).
tES generally uses very weak electrical currents (in the order of a few milliamperes [mA]). Furthermore, only a small fraction of the current reaches the brain, with its greater part being deflected across the skin, connective tissue, muscles, skull, meninges, and cerebrospinal fluid, which all can divert current before it reaches the brain. The aggregate of all the resistance to current flow presented by the head is referred to as impedance or resistance (
27,
37). Importantly, impedance (measured in ohms) then reflects the properties not only of the head but also of the electrodes in contact with the skin. In this way, when contact between the device and the head is poor at the electrodes, a high impedance may be detected by the tES device. High impedance levels are often associated with poor conductivity, reflecting a suboptimal electrode setup or humidification (
38). To avoid high impedances, the sponges should always be properly humidified but never in excess, for if the solution spreads over the scalp, the current will likewise spread over a large superficial area and will not flow properly across the desired brain regions (
13). The certified tES devices measure not only current intensity but also impedance and, for safety, warn the applicator or stop the current flow if too much impedance in the circuit is detected (
13).
Definitive and optimal tDCS parameters for psychiatric research and clinical practice are still in development, as these parameters may depend on patient characteristics (
39). Preliminary evidence suggests that increasing frequency of sessions, session duration, or current intensity might be associated with better clinical response (
39), especially with concurrent and directed mental activity (i.e., “target engagement” [
40]). Importantly, higher levels of total tDCS exposure (i.e., cumulative charge) have not been associated with greater adverse events (
41).
Basic knowledge of these parameters is a prerequisite to operate a tES device. Although tES devices (especially for tDCS sessions) are typically straightforward and safe (
14), it is important that certified equipment is used and that established protocols are followed by trained staff (
13,
15). Some devices are designed to be especially portable and simple to use (
42) and thus potentially adaptable for home use, provided there is proper patient training and remote supervision by clinical or research staff (
18,
43).
Mechanisms of Action
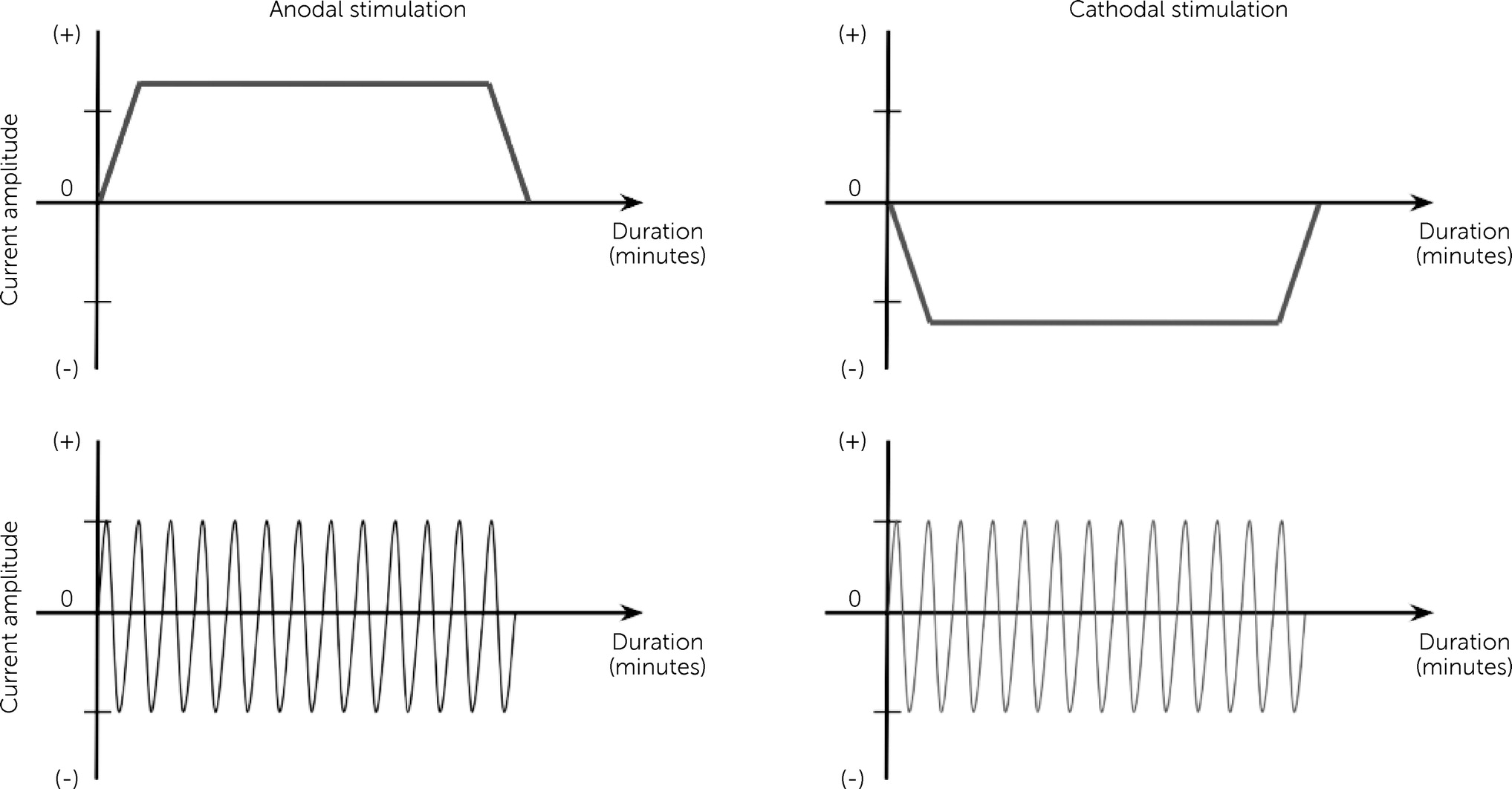
One main distinction that separates the tES modalities from one another is the waveform (temporal pattern) in which the electrical current is applied (
Figure 2). In tDCS, a continuous, direct electrical current of low intensity (e.g., typically up to 1–3 mA) passes through at least two electrodes (i.e., anode and cathode) that are applied noninvasively over the scalp (
14). tACS, unlike tDCS, delivers an oscillating, sinusoidal electrical current of low intensity (i.e., typically spanning 1–2 mA or less) to the brain via two electrodes applied on to the scalp, whose position and size are determined based on the target region of the brain (
29,
44). The method in applying conventional tACS is largely similar to tDCS, with the electrical current delivered via electrodes and saline-saturated sponges.
tDCS modulates neuronal activity on the network level by producing current flow around neurons, which results in an incremental shift in neuronal membrane potentials (e.g., polarization [
45]) and which, in turn, leads to a host of neuronal function changes, such as a change in firing rates (
20,
29,
46). These modulation effects may last after the stimulation period, potentially up to 0.5–2 hours, depending on the intensity and duration of stimulation due to changes in synaptic neuroplasticity (i.e., via mechanisms that resemble either long-term potentiation or depression) (
47,
48). In addition, the direction of current flow (i.e., parallel or perpendicular to the underlying pyramidal neurons of the stimulation sites [
49,
50]) is one of the key determinants of the desired neuromodulatory effects, along with dosage (i.e., current density and duration of stimulation), polarity, size, and placement of the electrodes (
19,
20).
tACS, in contrast, is considered to entrain neuronal oscillations to the stimulation frequency by producing an oscillating polarization of neurons (
51–
53). tACS may also produce changes in brain function outlasting the stimulation duration (
54–
56). During the half cycle of an AC oscillation, one electrode acts as the anode while the other acts as the cathode, and vice-versa for the other half cycle (
57).
Modulation achieved by tDCS and tACS is considered network based within the functionally connected regions beyond the superficial cortical regions, thus presenting more functionally specific stimulation, which can modulate task-relevant brain networks that may yield strong implications for clinical use (
12,
17,
19,
20,
58).
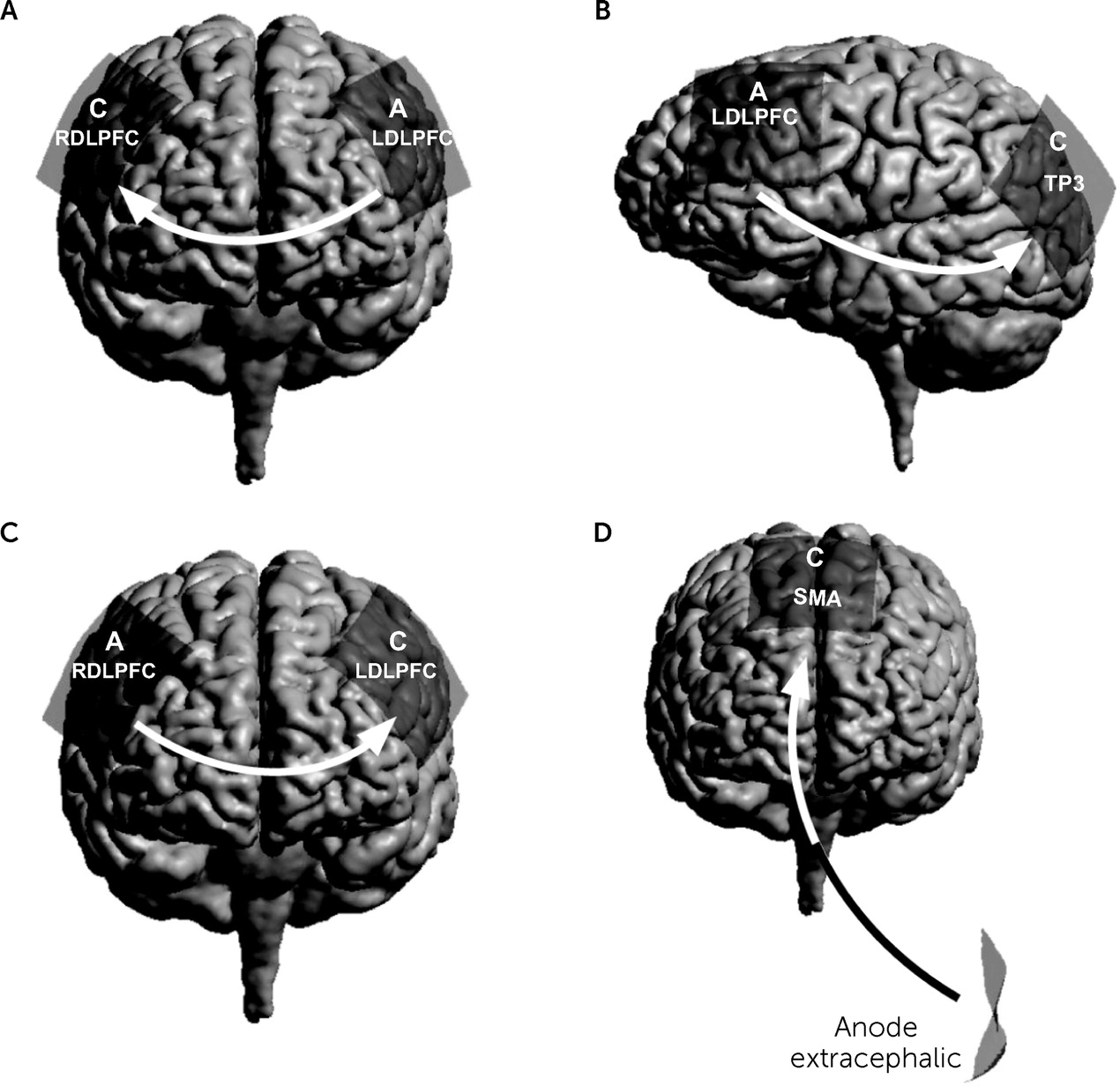
In summary, tDCS and tACS present unique neuromodulation options whether the goal is a more functionally or spatially specific target. These techniques are capable of modulating or stimulating task-related neural networks, and as such, they may allow modulation or normalization of dysregulated neural activity that is associated with specific psychiatric disorders. This review focuses on the use of tDCS in treatment of psychiatric disorders (
Figure 3) (
59).
Conclusions
The goal of this review was to provide an overview of recent advancements in tES research, with an aim to identify its appropriate application in clinical settings as an intervention for psychiatric disorders. Research using tES in clinical samples has grown exponentially over the past decade. This body of evidence reflects substantial safety and modest efficacy of tES in clinical practice, along with its potential to help patients with resistance to existing treatment options, increasing its clinical relevance. This review suggests that most promising clinical outcomes have been documented in major depressive disorder research, along with growing evidence of its application to treat schizophrenia, OCD, and substance use disorders. Furthermore, the review has demonstrated promising technological and methodological improvements in tES that allow for greater specificity in treating specific psychiatric disorders. Future research, using a precision medicine framework, should leverage cutting-edge neuroimaging findings to improve the mapping of disruptions in specific brain networks associated with distinct psychiatric disorders to better personalize tES treatment protocols. In all, the clinical use of tES is particularly alluring for its mild side effects and its potential to be used as an independent or combined treatment method. With better understanding of its impact on and interaction with brain functions, future research can provide more standardized guidelines that are specific to each psychiatric disorder, leading to improved clinical outcomes.