A leverage of these advances will depend on understanding the genetic influences and their interactions with the environmental context within which they operate. It is clear that there are multiple genes, signaling pathways, and interactions besides other modulatory factors such as age and sex. Moreover, it is known that environmental factors are quite influential.
14 Environmental factors are relevant even for highly heritable disorders such as schizophrenia, bipolar disorder, and autism and often have higher associated risk than common genetic variants for disorders such as anxiety and depressive disorders, with the exception of eating disorders.
15 Thus, it has been a major challenge to elucidate the mechanisms underlying how genes and related neural processes operate in response to environmental adversity.
Perhaps the best demonstrative series of examples come from the studies of the human serotonin transporter gene variation called the serotonin transporter–linked polymorphic region (5-HTTLPR) and stress sensitivity.
76 As already supported by recent data obtained from a GWAS, an association between 5-HTTLPR and major depressive disorder has been repeatedly suggested.
77 It was already reported in 1996 that this polymorphism in the promoter region of the human serotonin transporter gene (SLC6A4; also known as 5-HTT) regulates gene expression.
78 Both human and animal research supports the evidence for the effect of environment on the stress sensitivity in relation to genetic variation of 5-HTT.
79,80 Following this, several other studies addressed the modulatory impact of the 5-HTTLPR genotype on the effect of environmental variables.
81–84 (Although some replications of these studies were not satisfactory, it is speculated that small effect size has created a statistical artifact.
85) Supporting this, a moderate effect of the 5-HTTLPR genotype in relation to early life stress and levels of rumination has been shown by brain imaging studies.
86 Taken together, these data represent a demonstrative example of how data from animal research, behavioral genetics, imaging genetic studies, and integrated environmental intervention are beginning to unravel the interaction of genetic variation and environmental factors. However, levels of rumination may not be a good candidate to measure the pure effect of environmental factors because resilience is likely to be affected by both genetics and environment. Moreover, the integration of environmental factors into imaging genetics in this study was obtained by the classification of subjects based on self-reports, which might threaten the validity of the research. One further step from here might be an analysis of the effect of genetic variation on the neural processing of variable psychosocial conditions. Indeed, a progress targeting this comes from the data developed by multinational teams with projects clustered in the form of European Network of Gene Environment Interactions. Among these efforts, the neuroimaging study conducted by Lederbogen et al.
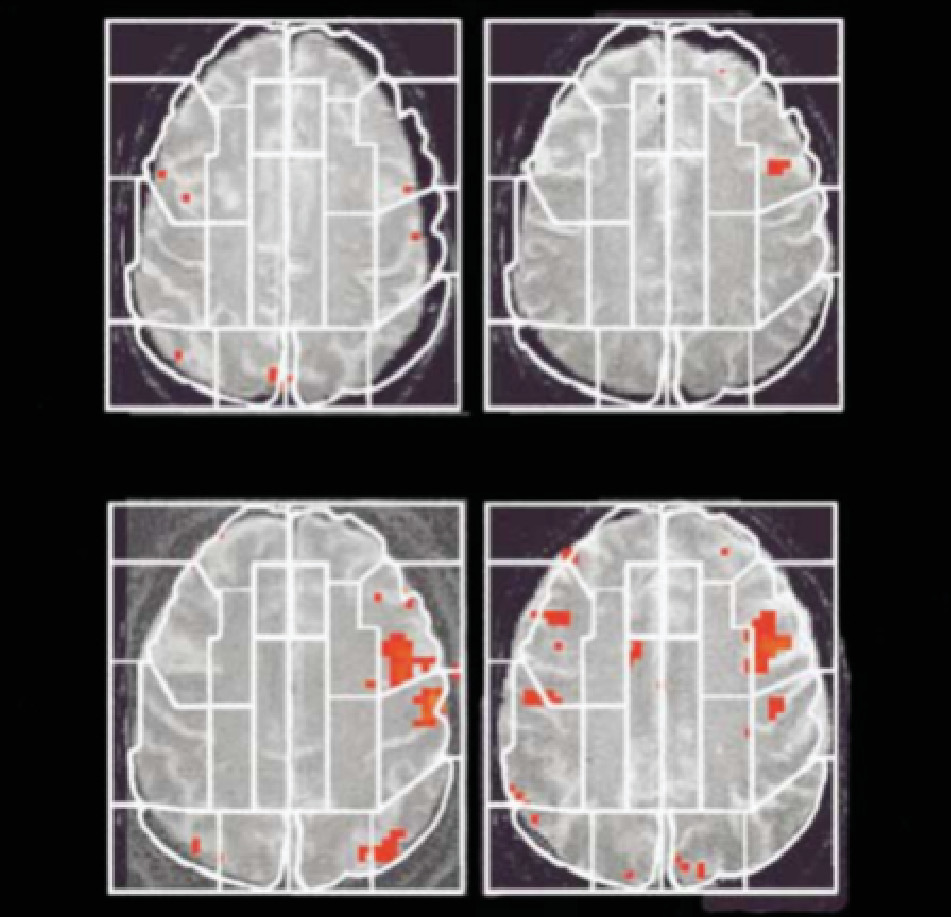
87 identified distinct neural mechanisms for an established environmental risk factor: urbanicity. At the first stage, participants were scanned when they were taking cognitive tests that they were told to fail. Moreover, to increase the stress level, the participants were provided with negative feedback through headphones while they were checked for stress indicators such as high blood pressure. Although there was no significant difference in the mental health between the two groups of participants, the way these two groups dealt with the stress was different, and that difference is targeted to two regions: the amygdala and the perigenual anterior cingulate cortex. These two regions were associated with increased activity for urban upbringing.
87 Although this study is not devoid of its own limitations (as described in the original paper and thus will not be repeated here), it is a good example of exploring the effect of environmental risk factors (urbanicity) to analyze the neural processing of psychosocial conditions (stress). Thus, the integration of epidemiological associations to understand underlying neural mechanisms affected by environmental conditions seems feasible by the use of neuroimaging.
A further analysis of this type would be achieved by the refinement of psychosocial components in urban contexts besides to a careful consideration of the role of genetic variation. Two studies appear to fill these gaps. First, neuroimaging experiments, conducted by Zink et al. in 2008,
88 have already begun to unravel how unstable social status, as an environmental risk factor, is mediated by specific neural mechanisms. The study provided a profile of the neural correlates associated with processing social hierarchies in humans. Animal studies already showed that the more subordinate position in stable social hierarchies is associated with greater stress, but in dynamic unstable social hierarchies, the dominant position is exposed to most of the stressors because of the increased competition and instability.
89 Thus, to address the neural correlates of social hierarchies in humans, Zink et al. analyzed the brain activity patterns of 72 Caucasian, right-handed, healthy adults in response to different hierarchical settings (stable and unstable) during a task (e.g., multiple rounds of simple reaction time tasks or a simple visual discrimination task). The tasks allowed the players to perceive the hierarchy by the use of other players simulated in the computer screen. Interestingly, the social unstable hierarchical setting produced neural activations, which were not detected in other settings. These areas (thalamus, amygdala, and posterior cingulate) are those linked to social emotional processing and overlap with the findings of Lederbogen et al.
87 Second, another stream of research with a focus on oxytocin merges with these data. Research across species has demonstrated that oxytocin plays a key role in the regulation of social cognition and behavior. As reviewed by Meyer-Lindenberg and Tost,
90 it plays a crucial role in attachment, social exploration, and social recognition, as well as anxiety and stress-related behaviors. Among the relevant candidates for oxytocinergic signaling, the most extensively studied candidate is the gene coding for the oxytocin receptor.
90 Among socio-behavioral phenotypes, SNPs in oxytocin receptor have been associated with positive mood,
91,92 sensitivity to social support,
93 deficits in mother’s sensitivity to her children’s behavior,
94 and reward dependence.
95 Particularly, an SNP of unknown functionality in the third intron of oxytocin receptor has emerged as an interesting candidate: rs53576 (G/A). Psychiatric relevance of this genetic variant manifests itself by studies indicating that rs53576A is overtransmitted in some families to offspring with autism spectrum disorders.
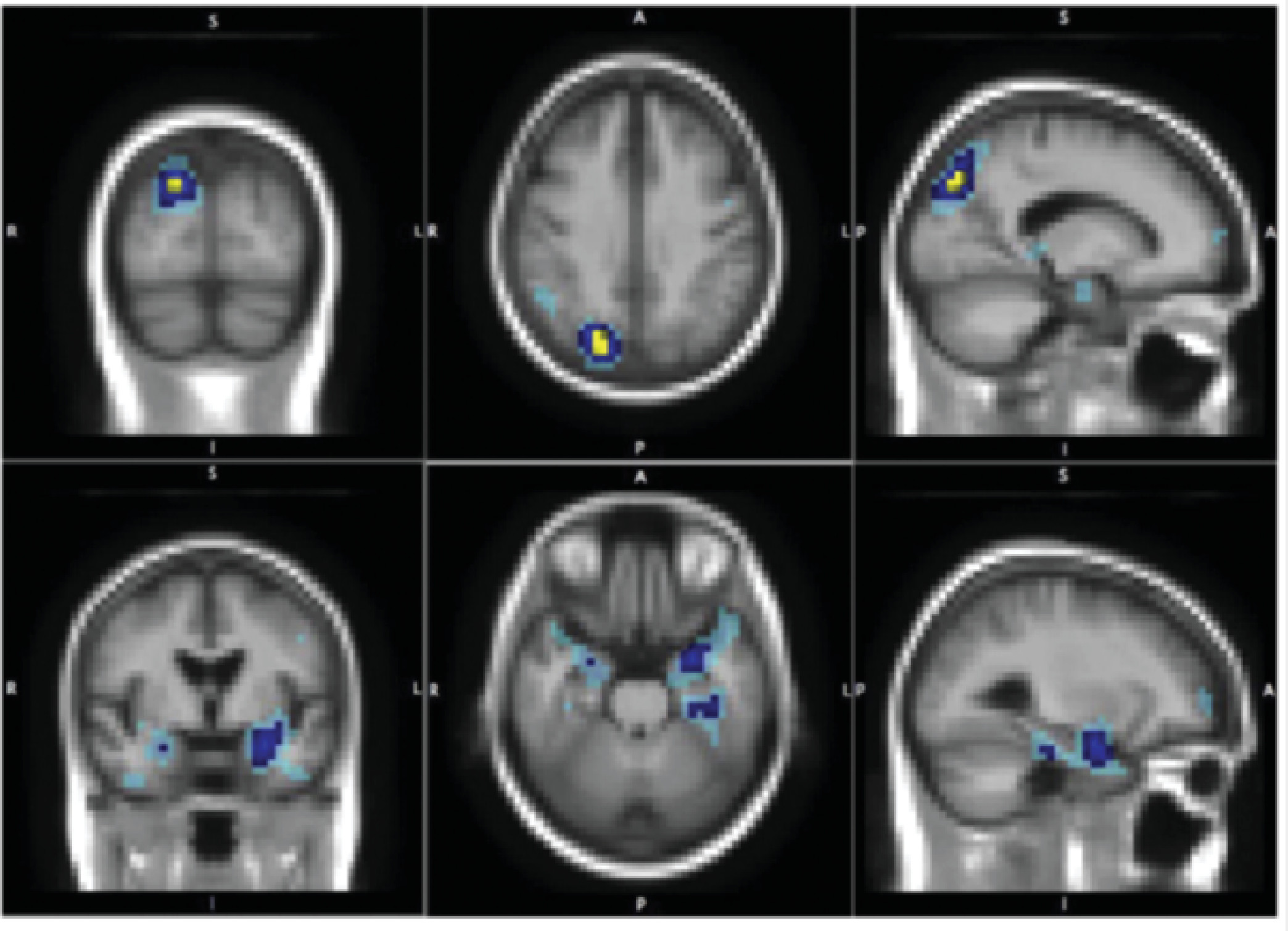
88 To address the intermediate neural mechanisms of this phenomenon, Tost et al.
96 used multimodal neuroimaging in a large sample of healthy human subjects to identify structural and functional alterations in oxytocin receptor risk allele carriers and their link to temperament. They found activation and interregional coupling of the amygdala during the processing of emotionally salient social cues, depending on the oxytocin receptor rs53576 genotype. In addition, they reported structural alterations in key oxytocinergic regions, which predict the lower levels of reward dependence, specifically in male risk allele carriers. Thus, in this example, accumulating studies show that oxytocin receptor gene variation (rs53576) is associated with structural and functional alterations in limbic circuitry involving the amygdala, the hypothalamus, and the cingulate gyrus, suggesting that it influences social cognition and behavior by modulating neural circuits for processing of social information and negative affect. The findings presented here converge on a previously defined circuit, the amygdala cingulate circuit, modulated by the serotonin system–related gene variant, 5-HTTLPR, which is characterized as susceptibility to environmental adversity in relation to the risk of depression,
82,97,98 as already mentioned above. Taken together, these suggest that the amygdala cingulate circuit is a neural substrate, a form of neurobiological cluster, exposed to the effect of divergent molecular factors (i.e., of serotonergic and oxytocinergic systems impacting on environmental reactivity and acting as a functional unit that might play a major role for the pathophysiology of relevant psychiatric conditions). Thus, these data provide evidence for the utility of imaging genetics studies—when combined with data from different sources—in analyzing the effect of genetic variation and environmental factors on neural processing of social conditions as a susceptibility mechanism for psychiatric conditions, in this case via a mechanism dependent on the medial prefrontal cortex and amygdala.