The concept of volume transmission as an important mode of chemical communication in the brain was introduced in the 1980s.
1,3,17–20 New techniques (e.g., receptor autoradiography, immunohistochemistry) that allowed detailed mapping of the locations of neurotransmitters (NTs) and their receptors led to several experimental findings that were not compatible with a purely synaptic mode of operation. Mapping studies found relative mismatches in some brain areas between the density of nerve terminals positive for a particular NT and the density of receptors for that NT. Situations in which there were very few terminals, as compared with the number of receptors, indicated that active substances must be getting to receptors in some manner other than release from an adjacent terminal. Studies demonstrating presence of NTs outside of synapses under several sets of experimental conditions (e.g., release enhanced, reuptake blocked) indicated that spillover from the synaptic cleft into the surrounding extracellular space (ECS) was one possible source.
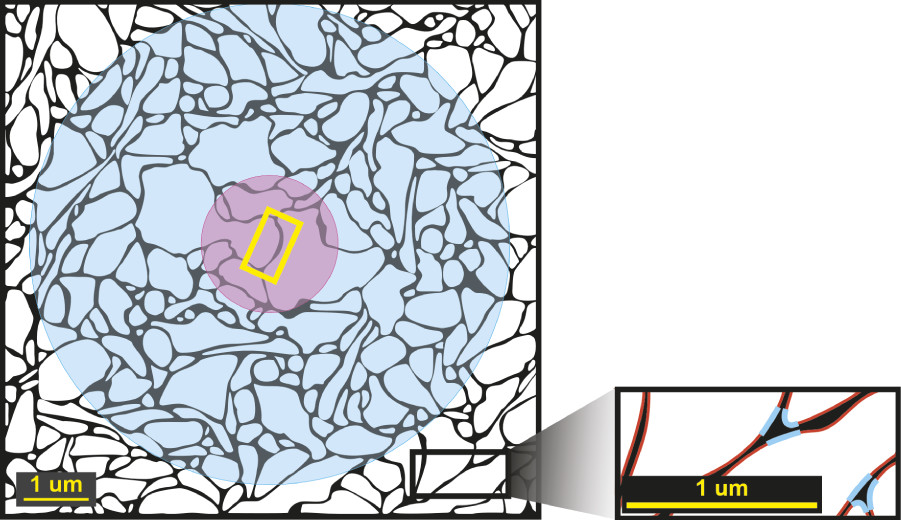
2 After release, diffusion of NTs through the ECS is likely to be sufficient to activate nonsynaptic receptors for a considerable distance (
Figure 1).
1–3 Mapping studies have identified axon terminals without postsynaptic specializations. Also, varicosities containing granules filled with monoamine NTs have been localized, not only in axon terminals, but also in other portions of neurons (e.g., dendrites, soma, non-terminal axon segments). These findings support the possibility of nonsynaptic release of NTs into the ECS.
1,3,20 Nonsynaptic release was also supported by the finding of measurable levels of specific NTs in the ECS in regions lacking synaptic terminals for that NT.
20 On the basis of animal (primarily rodent) studies, it was concluded that the modulatory NTs (e.g., acetylcholine, norepinephrine, dopamine, serotonin) act in great part by volume transmission because the majority of their NT-containing varicosities are not part of a synapse, and many of their projections do not make synaptic contact.
1 This finding is supported by a recent study in nonhuman primates, reporting that 94% of tyrosine hydroxylase-positive boutons in prefrontal cortex Area 10 had no identifiable synaptic structures, indicating primarily nonsynaptic release of dopamine.
21 There may be species-specific differences, as one study found a much higher percentage of acetylcholine-containing varicosities associated with synapses in the human cerebral cortex (67%) than had been reported for the rat (15%).
22 Mechanisms for release of NT from nonsynaptic varicosities include exocytosis (similar to synaptic release) and reverse operation of NT transporters.
1,17–20 Many other neuroactive substances (e.g., glial transmitters, peptides, nitric oxide, neurosteroids) are also released into the ECS.
1,17–19 There is growing recognition that volume transmission is an important mode of action for other NTs (e.g., glycine, glutamate, GABA), as well.
23–26 The location of a receptor, compared with the source of the neuroactive substance, is likely to be very important in determining activation.
1–3 Many receptors that are not part of a synapse are located sufficiently far from the synaptic cleft that they will be unable to detect individual release events. Rather, they will experience a summation of surrounding events. Thus, they are more likely to signal slower (tonic) changes that are occurring over a larger area.