Schizophrenia is a heterogeneous disorder characterized by significant abnormalities in the brain resulting in various symptoms such as delusions, hallucinations, disorganization, deficit symptoms, and language abnormalities in addition to neurocognitive deficits.
1 The symptom profile of this disorder has been meaningfully conceptualized as variable occurrence of positive symptoms (delusions and hallucinations), negative symptoms (e.g., apathy, affective blunting, and lack of socialization), and disorganization symptoms.
2 The positive symptoms of schizophrenia have been a wide area of research, and the temporal lobe forms one of the important substrates for these symptoms.
3 Abnormalities of the temporal lobe have been described in schizophrenia, and robust pathological aberrations have been reported in the superior temporal gyrus (STG).
4,5The lateral surface of the temporal neocortex, predominantly the STG, is considered to be a major anatomical substrate for speech and language functions.
6 The STG has connections with many areas implicated in schizophrenia such as the limbic system, thalamus, and prefrontal cortex.
7 Evidence suggests the the STG plays an important role in the pathophysiology of schizophrenia symptoms. The superior part of the STG contains the auditory sensory cortices: the Heschl’s gyrus (HG) and planum temporale. Previous studies have demonstrated significant volume reductions in STG, especially on the left hemisphere.
8 In addition, loss of the normal planum temporal asymmetry has been reported.
9,10 It is possible that positive symptoms like hallucinations may be mediated by abnormalities in the auditory cortex and the related auditory processing areas.
11,12 Through functional MRI studies, it has been shown that occurrence of auditory verbal hallucinations has been associated with activation of temporal lobe structures.
13,14 Involvement of primary auditory areas in auditory verbal hallucinations was reported by Dierks et al in a functional MRI study, demonstrating an increase of the blood oxygen level–dependent signal in HG during the hallucinations.
14 Reduced left and increased right temporal cortical response to the auditory perception of speech has been reported in schizophrenia in a functional MRI study demonstrating that the auditory hallucinatory state is associated with altered activity in temporal cortical regions.
15In view of this, volumetric examination of the STG and HG might contribute importantly to our understanding of the pathophysiology of schizophrenia. We hypothesized that medication-naïve schizophrenia patients will have a volume reduction in STG and HG compared with matched healthy controls (HC). In addition, we hypothesized that volume reduction of temporal lobe structures would have correlation with schizophrenia psychopathology severity, especially with the positive symptoms. With this hypothesis, we aimed to examine the volume of STG and HG in antipsychotic-naïve adult schizophrenia patients in comparison with HC. We also sought to examine the relationship between the volume alterations and symptom severity of schizophrenia.
Methods
Subjects
The sample consisted of 55 antipsychotic-naïve patients with schizophrenia and 45 healthy comparison subjects. The patients with schizophrenia were recruited from the outpatient services of the National Institute of Mental Health and Neurosciences, Bangalore, India. The healthy control subjects were consenting volunteers recruited to ensure age and sex matching as a group. The institute ethics committee clearance for the study was obtained. All subjects were recruited only if they provided the informed written consent. All the subjects were of Indian origin/ethnicity.
Only right-handed subjects (patients and control subjects) were included in this study.
16 DSM-IV diagnosis of schizophrenia
17 was established using the Structured Clinical Interview for the DSM-IV.
18 The diagnosis was confirmed through independent clinical interview by an experienced psychiatrist (G.V.S.). The subjects with a history of a first episode and illness duration as defined by report of psychotic symptoms were assessed using the Instrument for the Retrospective Assessment of Onset of Schizophrenia.
19 None of the patients were exposed to any psychotropic medications including antipsychotics before the assessments.
Subjects did not have a history of medical illness, substance dependence, or comorbid axis I disorders. No subject had any contraindications to MRI (cardiac pacemaker, aneurysm clip, cochlear implants, pregnancy, intrauterine device (IUD), history of metal fragment in eyes, neuro-stimulators, weight of 250 lbs or more, claustrophobia). None of the subjects had any medical illness that may significantly influence CNS function or structure, significant neurologic disorder such as seizure disorder, cerebral palsy, or history suggestive of delayed developmental milestones (suggestive of mental retardation), family history of hereditary neurologic disorder that may complicate diagnosis, comorbidity for DSM-IV psychoactive substance dependence, or lifetime history of head injury associated with any of the following: loss of consciousness longer than 10 minutes, seizures, neurological deficit, depressed skull fracture, surgical intervention, or CNS infection. Female subjects were neither pregnant nor within the postpartum period.
Assessment of Psychopathology
The psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS).
20 Interrater reliability for psychopathology scores was examined with another qualified psychiatrist. The ratings were simultaneous when one of the raters (in turns) examined a series of 15 patients. The interrater reliability was calculated using the intraclass correlation coefficient. The intraclass correlation coefficients for positive syndrome, negative syndrome, and general psychopathology were >0.9, indicating excellent interrater reliability.
Healthy comparison subjects were screened using the 12-item General Health Questionnaire
21 and a comprehensive mental status examination. None of the healthy comparison subjects had a family history of psychiatric illness in their first-degree relatives. None of the subjects (patients or healthy control subjects) scored positive for alcohol use on the CAGE questionnaire.
22 None used stimulants, opiates, or cannabinoid drugs. No subject had a history of a neurological/medical disorder. In addition, no subject in the study had abnormal involuntary movements.
MRI Acquisition
MRI was done with a 1.5-T scanner (Magnetom Vision; Siemens, Erhlangen, Germany). T1-weighted three-dimensional magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence was performed (TR=9.7 ms, TE=4 ms, nutation angle=12°, and slice thickness 1 mm with no interslice gap) yielding 160 sagittal slices.
Volumetric Method
T1-weighted images were processed using SPM8 (Wellcome Trust Centre for Neuroimaging, UCL, London; UK;
http://www.fil.ion.ucl.ac.uk/spm) implemented in the voxel-based morphometry Toolbox 8 (
http://dbm.neuro.uni-jena.de/vbm.html) under MatLab 7.8.0 (The MathWorks, Sherborn, MA). Standard routines and default parameters of the voxel-based morphometry 8 toolbox were applied. After setting the image origin to the anterior commissure, images were bias corrected, preregistered to standardized International Consortium for Brain Mapping (East Asian brains) space using affine transformation with regularization, and segmented using the unified segmentation approach. SPM8 segmentation was based on a modified Gaussian mixture model to avoid misclassification. A spatial adaptive nonlocal means filter was applied to remove noise while preserving edges. To remove isolated voxels of one tissue class within a cluster of voxels belonging to a different tissue class, a hidden Markov random field model weighting was used. Spatial normalization was performed with high-dimensional Diffeomorphic Anatomical Registration using the Exponentiated Lie algebra template.
23 Gray matter segments were modulated by the Jacobian determinants of the deformations to account for local expansion and compression introduced by nonlinear transformation. Finally, the gray matter images were smoothed with an 8-mm full-width at half-maximum isotropic Gaussian kernel. This was done to reduce errors related to intersubject variability in local anatomy and to render the imaging data more normally distributed. A priori regions of interest by masks were created using the Wake Forest University School of Medicine Pickatlas.
24Gray matter, white matter, cerebrospinal fluid, and total intracranial volumes were calculated as previously described.
25 Apart from examining the group differences, statistical parametric maps were also examined for correlation between volumes (STG and HG) and positive and negative syndrome scores (corrected for total PANSS score). The general psychopathology score was not used for correlation analysis because of its inherent item heterogeneity.
Statistical Analysis
The clinical data were analyzed using the independent-samples t test and chi-square test.
The comparisons were done between patient group and healthy control subject group using the independent-sample t test model using SPM with correction for multiple comparison using the family-wise error correction method (p<0.05). For the psychopathology correlation analysis, an uncorrected p value of 0.001 with control for multiple comparisons by 10-mm sphere small volume correction method was applied. The MNI coordinates (SPM space) were converted to Talairach atlas space using the MATLAB code “mni2tal.” Stereotactic coordinates are reported in Talairach atlas space,
26 and regional localizations were ascertained along with the Brodmann’s areas (BAs).
Discussion
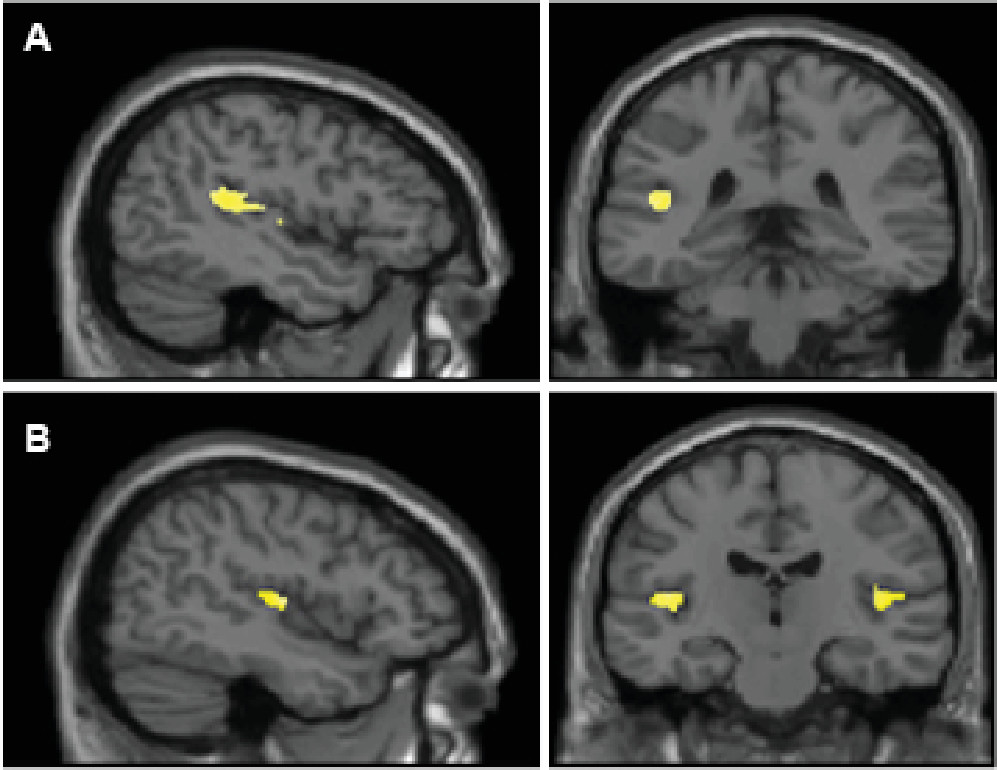
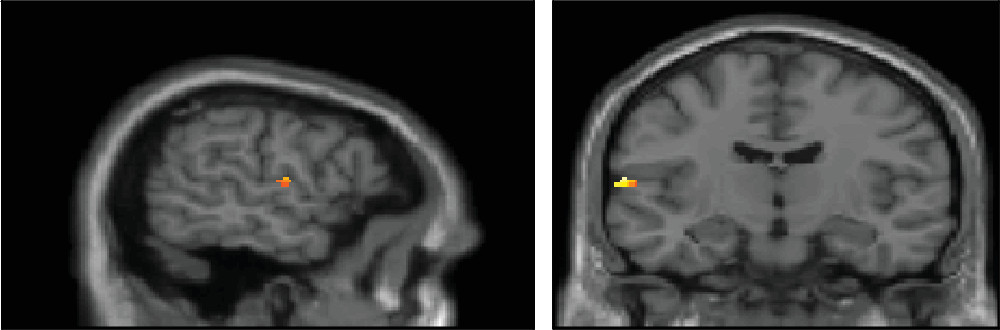
This is one of the largest studies to date on antipsychotic-naïve patients with schizophrenia examining STG and HG volumetric abnormalities. The present study emphasizes the role of temporal lobe cortical areas in the pathophysiology of schizophrenia. There was a significant reduction in the volume of HG bilaterally compared with healthy control subjects. The volume of the left STG was found to be reduced significantly compared with healthy control subjects. In addition, PANSS positive symptom score negatively correlated with left HG.
STG is involved in auditory polymodal sensory information processing in addition to language functions and auditory memory.
27,28 The finding of STG gray matter volume reduction among patients with schizophrenia may be related to auditory and related abnormalities in higher cognitive function in schizophrenia. Volumetric abnormalities of HG have been examined earlier, although less frequently, compared with the studies on volume of STG as a whole. In a previous study, it was reported that HG on either side were smaller in patients with first-episode schizophrenia (relatively short duration of illness) compared with healthy control subjects.
29 However, a longitudinal study demonstrated a rather specific reduction of the left HG over time.
30Because the HG is the primary auditory reception area, it plays a crucial role in the initial processing of auditory information. Hence, its volume reduction bilaterally in schizophrenia can be explained because of the importance of auditory hallucinations in the pathology of schizophrenia. This finding raises a potential possibility that there could be primary auditory sensory deficits in schizophrenia. In this regard, patients with schizophrenia performed poorly compared with control subjects in low/high tone discriminating ability against a background noise in a previous report.
31 This is further substantiated by the deficits in mismatch negativity (MMN) in patients. HG is the generator of MMN, which represents an event-related potential of early auditory processing.
32 This parameter was found to be reduced in schizophrenia.
33 In addition, a functional MRI study showed reduced activation of bilateral HG in response to auditory mismatch stimuli.
34STG on the left side (dominant hemisphere) has important language processing functions. An important component of STG, namely the planum temporale, depicts the most prominent left-right asymmetry of the human brain.
35 This could be related to its language processing role in humans.
36 Volume reduction of STG on the left side as demonstrated in this study would result in the loss of this normal asymmetry and might be responsible for auditory processing and language abnormalities found in schizophrenia. Indeed, a reduction in STG volume in schizophrenia has been reported in some previous studies, including a progressive loss of gray matter volume of this area.
8,37,38The exact neurobiological mechanism responsible for the volume change in the left STG is yet to be definitively ascertained. However, there is evolving evidence implicating abnormal excitatory amino acid neurotransmission in schizophrenia perhaps occurring through an insufficiency in recurrent inhibition.
39 This mechanism could be a likely cause of ongoing damage in the course of excitotoxic effects. However, it is difficult to substantiate a left-sided volume reduction with this explanation. It could arguably be due to the fact that left and right STG have some disparity in the cytoarchitechture,
40 and this may be vital in the mechanisms for lateralized changes. STG and HG could be mediators of positive symptoms as evidenced in the present study results. According to the previous literature, STG has been found to be involved in the production of hallucinations. Volume reduction of the STG correlated with the severity of hallucinations,
41 delusions,
42 or positive symptoms in general
11 according to the previous literature.
This study has a major advantage of studying antipsychotic-naïve patients with schizophrenia. This is among the largest studies on an exclusively antipsychotic-naïve patient sample examining the volumetric abnormalities of the temporal lobe in schizophrenia. Antipsychotic medications can produce volumetric changes in the brain. Region-specific volume changes with antipsychotic medications have been documented even over a very short period of time.
43 The other advantages of this study are a large sample size and structured assessment of psychopathology using standard scales. In addition, we used an automated and efficient brain analysis to detect structural differences, thus eliminating bias by the investigators. Even though the subjects had a mean duration of illness of 16 months, which can contribute to the illness progression after the inception of biological changes, they were still in a relatively early phase of illness. This could imply that the findings in this study may be due to neuro-developmental abnormalities even though there can be contributions due to illness variables and duration. However, the cross-sectional design of our study does not allow drawing clear inferences on the timing of these volume changes and the likely changes during the course of the disorder. Finally, there are limitations inherent to the technique of voxel-based morphometry, such as sensitivity to method-related options in normalization, smoothing, and template. The hypothesis-driven feature of the a priori region of interest method could result in overemphasis on the biological changes in a prespecified region without taking into account the changes that could have been present in other brain regions.
In conclusion, this study highlights the importance of temporal lobe pathology in schizophrenia and the relationship between these substrates and the psychopathology in schizophrenia. In appears intuitive that the psychotic symptoms may not be associated with a single regional deficit. Rather, several regions of a more intricate circuitry might be involved. However, it appears that changes in the primary auditory cortex and temporo-parietal areas could form the core of this aberration. There is a need to examine the functional correlates of temporal lobe abnormalities in schizophrenia in larger samples of drug-naïve patients. The structural and functional abnormalities of the temporal lobe when coupled with electrophysiological measures such as MMN and P300 in antipsychotic-naïve subjects could help elucidating biological substrates for positive psychotic symptoms. In addition, progressive brain changes using imaging with a longitudinal study design could be very informative regarding the course of brain changes and its relation with symptom measures. Above all, temporal lobe is an important target for brain stimulation techniques like repetitive transcranial magnetic stimulation
44 and transcranial direct current stimulation
45 in the treatment of refractory-positive symptoms. Studying the underlying changes in the brain plasticity with these interventions might result in a better understanding of the neurobiological underpinnings of schizophrenia, with a particular relevance to the role of temporal lobe.