To the Editor: Normal pressure hydrocephalus (NPH) is caused by a disturbance of CSF circulation with normal CSF pressure.
1–5 NPH can present in two forms: primary (also known as idiopathic), for which no causative disorders are known, and secondary, for which other disorders such as trauma, infection, and hemorrhage are known causes.
1–5 Although the classic triad of subcortical dementia, gait apraxia, and urinary incontinence is the most common, there are several other clinical pictures, including tremor, appendicular ataxia, psychiatric manifestations such as anxiety and psychosis, and even oligo-symptomatic cases.
1–5 Other evidence has indicated that NPH is associated with sleep disorders, such as apnea.
6,7 When the physiopathology of NPH involves pathologic intracranial pressure, it may interfere with central breathing. We report a case of improvement of central sleep apnea in a patient with NPH after CSF shunt.
Case Report
We report a case of a 62-year-old white man who presented for neurosurgical evaluation because of impaired memory and slowing gait for 1 year. He was a functional high school teacher in the public educational system in Brazil. His medical history revealed arterial hypertension and smoking (45 packs/year). He had suffered acute myocardial infarction 3 years earlier. In addition, he had undergone routine pneumology evaluations during the previous 3 years because of snoring, daytime sleepiness, and a sleep disorder characterized by central apnea (not Cheyne–Stokes respiration) and severe apnea/hypopnea index (
Table 1). He used a continuous positive airway pressure (CPAP) device regularly to address his obstructive apnea but continued to have a high number of central apnea episodes.
His clinical examination was unremarkable. A heart examination revealed incipient diastolic dysfunction, with an ejection fraction of 62% (normal, 55%) and a left atrium diameter of 4.3 cm (normal, 2–4 cm). His body mass index was 31 kg/m2, and he was classified as Mallampati III.
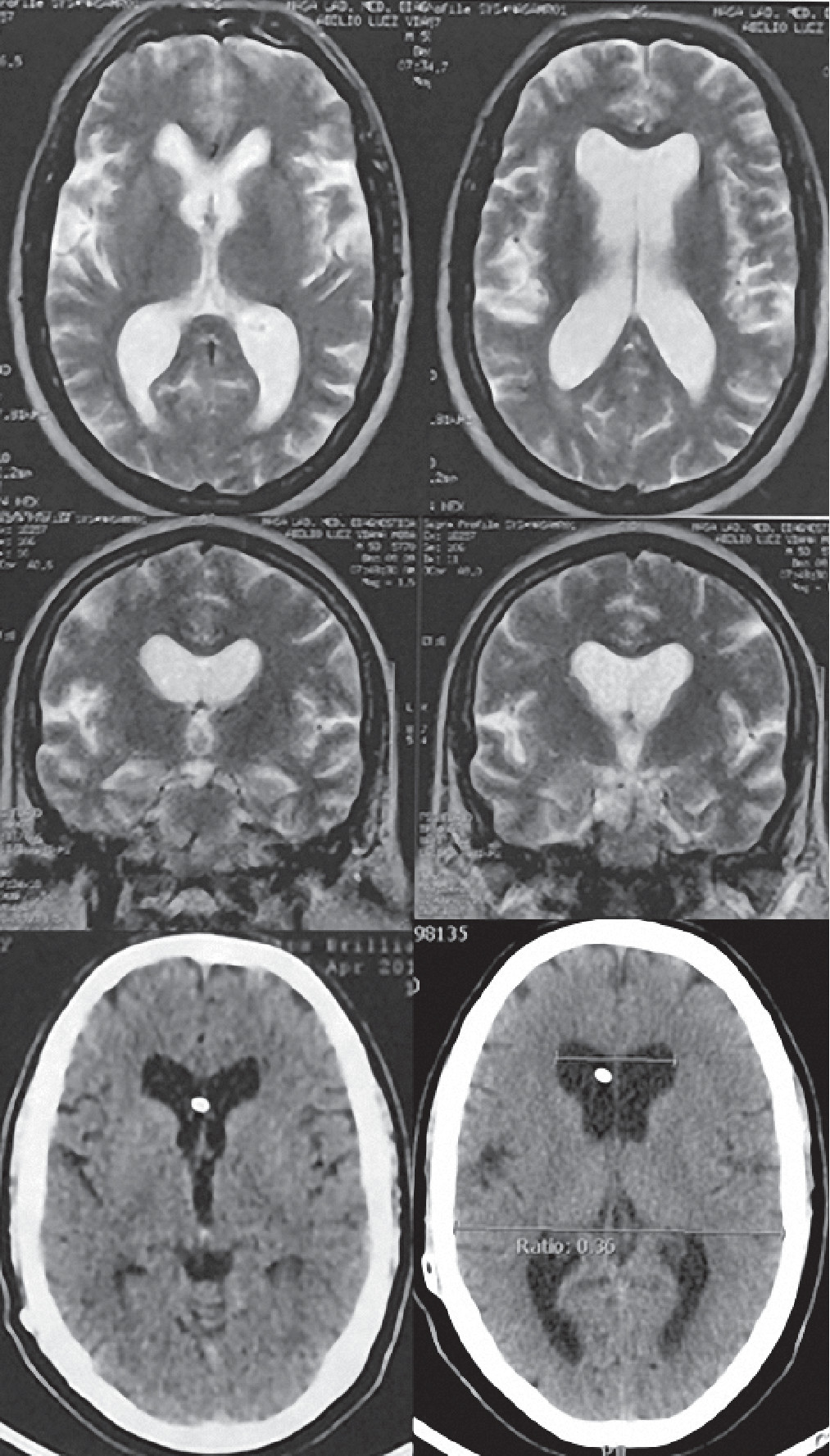
A neurological examination revealed a Glasgow Coma Scale of 15 and no deficits. The patient reported that he was experiencing gait apraxia, but he had a Timed Up and Go (TUG) of 12 seconds (normal, <20 seconds). His Mini-Mental State Examination (MMSE) score was 27/30, losing points for memory skills. His Japanese score for NPH (JSNPH) was 4. An MR examination revealed ventriculomegaly with an Evans ratio of 0.38 (
Figure 1). After undergoing a Tap Test, his results revealed a better TUG (10 seconds), a higher MMSE score (28/30), and a lower JSNPH (3).
At that time, the mandatory diagnosis of idiopathic normal pressure hydrocephalus and treatment with ventriculoperitoneal shunt was proposed.
Surgery was performed with no complications, and the patient was forwarded for ambulatory basis. Three months after surgery, he repeated polysomnography, which revealed an impressive and surprising decrease in central apnea occurrence (
Table 1). He is currently in the first year of follow-up, in which he repeated polysomnography with maintained patterns. CT examination of the skull revealed a functional shunt with an Evans ratio of 0.36 (
Figure 1). His most recent clinical evaluation revealed an MMSE score of 28/30, a TUG of 11 seconds, and a JSNPH of 2. Currently, he reports amelioration of daytime sleepiness and snoring with better cognitive and motor skills.
Discussion
Our case illustrates the improvement of central sleep apnea after shunting in an NPH patient. The patient complained of mild dementia and gait apraxia. His previous medical history included arterial hypertension, smoking, myocardial infarction, and sleep breathing disorder resulting in use of CPAP. We discussed the role of NPH in inducing central apneas and the reversibility of symptoms after shunting.
The pathophysiology of NPH is still unclear, but there is probably a low complacency in the frontal cortex, basal ganglia, and thalamus.
1–5,7 Although the mean intracranial pressure is within normal limits, its qualitative analysis is characterized by Lundberg
B waves with an amplitude of up to 50 mmHg, especially at night, which is probably related to different sleep stages, apnea episodes, and rhythmic alterations of cerebral blood flow.
6,8,9 Thus, this high-resistance and low-complacent environment promotes hypoperfusional deficits and interstitial edema, both of which are associated with apnea and other sleep disorders.
10–14The characteristics of sleep profiles in patients with NPH have been reported scarcely. For young adults, delta sleep corresponding to sleep stages 3 and 4 is rare in most patients, in addition to a lack of REM sleep and frequent awakenings during the night.
8,9,15 Until now, most reports in the literature have shown that mainly obstructive apnea (and not central apnea) is associated with NPH and that it is not ameliorated by lumbar CSF drainage or shunting, suggesting nonreversible neurological damage induced by hydrocephalus.
6,8,9,12Nevertheless, Kuchiwaki et al.
8,9 performed polysomnography in six of their patients 4–15 months after shunt operations. Five patients were classified as idiopathic cases, and one patient had NPH secondary to head trauma. A marked reduction of apnea was noted in several patients.
We highlight the potential association of NPH with sleep disorders on the basis of its pathophysiology. Central sleep apnea was specifically reversible after shunting, suggesting brain resilience after removal of a mechanical factor. In addition, we believe that NPH patients should have their sleep profiles evaluated routinely from the point of diagnosis through the postoperative period if it is determined that this finding is more frequent than once thought. Finally, patients with sleep disorders should also be evaluated by a multidisciplinary team composed of pneumologists, otorhinolaryngologists, neurologists, and even neurosurgeons because of the association of the different mechanisms in the genesis of such a condition.