In Tourette’s syndrome (TS), repetitive, unwanted movements (i.e., motor tics) and vocalizations (vocal tics) may in part be related to ineffective inhibitory control of strong urges to act. Reductions in the proficiency of inhibitory motor control are commonly measured across a range of neuropsychiatric conditions linked to aberrant frontal-basal ganglia circuitry function.
1,2 TS has also been linked to alterations in these circuitries, which has motivated the idea that characteristic motor and vocal tics seen in TS may involve a fundamental disruption to inhibitory control mechanisms.
3Evidence that TS alters inhibitory motor control processes is quite mixed. One reason for the variable findings is high co-occurrence of neuropsychiatric symptoms, such as attention-deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD), that are also linked to inhibitory control dysfunction.
2,4 Inadequate control of these comorbidities can confound study results, and few studies have directly investigated how these conditions interact to influence inhibitory control. Additionally, a handful of studies in children with TS report normal, or even more proficient, inhibitory control that is paralleled by greater activation of prefrontal brain regions implicated in cognitive control processes.
5,6 This has led to the conjecture that the persistent efforts to control tic behavior in childhood and early adolescence may actually contribute to the development of an overcompensatory inhibitory control system.
7,8 Moreover, many individuals with TS show remission or significant reduction of tic behavior in late adolescence and early adulthood around the same time that inhibitory control mechanisms fully mature,
9 although no studies have directly investigated longitudinal changes in inhibitory control in individuals whose symptoms remit.
At first glance, these findings are not easily reconcilable with the idea that TS is accompanied by a generalized disruption to inhibitory control mechanisms. Alternatively, inhibitory control dysfunction in TS may be a key feature in subgroups of individuals who either show certain neuropsychiatric comorbidities or, as hypothesized by some in the literature, display an unremitting clinical course into adulthood.
10,11 One study showed that smaller caudate nucleus volumes in childhood predicted greater motor tic severity in adulthood, and several additional studies have reported abnormalities in prefrontal and basal ganglia circuitries among adults with persistent TS symptoms.
12,13 These patterns suggest that some patients with TS may experience specific alterations in frontal-basal ganglia structures that are also implicated in inhibitory control mechanisms. In support of this idea, we showed that young adults with unremitted, persistent TS were less proficient at suppressing prepotent, impulsive actions in a response conflict task.
14 Additionally, Goudriaan and colleagues
15 reported that adults with TS were slower at stopping already initiated movements, and this deficit persisted even after excluding individuals with neuropsychiatric comorbidities.
Here we extend this work by investigating inhibitory control in groups of primarily young adults (and a handful of late adolescents) with persistent, unremitting TS and healthy controls. Like Goudriaan and colleagues,
15 we used a gold standard measure of inhibitory motor control, the stop-signal task.
16 We studied individuals with TS relatively uncomplicated by neuropsychiatric symptoms. Stopping, or inhibition, of initiated actions has been linked to the same prefrontal-basal ganglia circuitries putatively disrupted in adults with TS, thus providing a powerful test of the inhibitory control hypothesis of TS.
10,17 Notably, prior studies of inhibitory control in TS have focused exclusively on inhibition of manual responses. As a key novel component of our study, we investigated inhibitory control of both
manual and
vocal responses.
18 Given the hypothesized disruption of prefrontal-basal ganglia inhibitory control circuits associated with this subgroup of individuals with TS, we predicted poorer inhibitory control (i.e., delayed stopping speed) over manual and vocal actions.
Methods
Participants
Fifty-two participants, including 26 diagnosed with TS and 26 participating as healthy controls (HC), were enrolled through a specialized movement disorders clinic at the University of Virginia and community advertisement. The two groups were similar in age, education, and gender (see
Table 1). All participants were initially screened for a diagnostic history of ADHD or OCD as well as current symptoms of major mood or anxiety disorder. Participants with no diagnostic history of ADHD or OCD, and no current report of untreated major mood disorder were enrolled in the study. At study visit, a neurologist specializing in movement disorders (DC) confirmed the clinical diagnosis of TS according to guidelines contained in the
DSM-IV. All TS patients reported motor and vocal tics with onset prior to age 18. Eight of the patients had a diagnosis of TS that included a history of vocal and motor tics, but reported that vocal tics, but not their motor tics, had subsequently remitted (with increasing age).
Of the 26 TS participants, six were taking medications to treat tic symptoms, including atypical antipsychotic (N=2), tetrabenazine (N=2), and clonidine (N=3). Ten were taking either a serotonin reuptake inhibitor (N=6) or tricyclic antidepressant (N=4) for a past diagnosis of depression, and all reported normal mood functioning and denied serious depressive symptoms during interview and based on questionnaire data. None met
DSM-IV criteria for major mood disorder. All had normal or corrected-to-normal vision, denied color blindness, and provided informed consent before study participation. The study was approved by the University of Virginia institutional review board (protocol #15042), and all subjects signed a written informed consent document. Many participants also completed a response conflict task reported elsewhere.
14Screening Measures
All completed the American National Adult Reading Test
19 to estimate verbal intelligence as well as questionnaires to assess depression (Beck Depression Inventory),
20 anxiety (Beck Anxiety Inventory),
21 OCD (Yale-Brown Obsessive Compulsive Scale),
22 and ADHD (Conners’ Adult ADHD Rating Scales-Short Version).
23 Additionally, TS participants completed the Yale Global Tic Severity Rating Scale (YGTSS).
24Manual Stop-Signal Task
The latency of inhibiting initiated actions was measured with the stop-signal task.
16 In this task, participants perform a series of speeded choice reactions to go stimuli, but try to inhibit these reactions when an occasional stop signal appears shortly after the onset of the go stimulus. The task provides a sensitive metric of how much time an individual needs to stop a reaction that is being prepared for execution, aptly termed stop-signal reaction time (SSRT).
The manual version of the stop-signal task required participants to make speeded button presses to a series of directional arrows presented in the center of a computer monitor. Each trial began with the presentation of a single green-colored arrow that pointed to the left or to the right. Participants were instructed to press a button on the end of handheld grips with their left or right thumb based on the direction indicated by the arrow (e.g. left pointing arrow=left button press), and encouraged to respond quickly and accurately. After a button press or a lapse of 1,200 ms, the arrow disappeared, and a random interval ranging from 1,200 to 1,700 ms transpired before the onset of the next green arrow. A fixation point remained on the screen during the interstimulus interval to help participants focus their visual attention on the location of arrow presentation. On 25% of the trials, the green arrow changed color to red shortly after its onset. Participants were instructed to stop their button press when the arrow turned red (stop trials). The timing of the delay between the onset of the green arrow and the onset of the color change (stop-signal delay [SSD]) was set initially at 200 ms and then adjusted dynamically across stop trials using a staircase-tracking procedure that controlled for the success of stopping (i.e. inhibition probability).
25 Specifically, following a successful stop, the next stop-signal delay was prolonged by 50 ms, thus making it more difficult to stop. On stop trials in which a response was not stopped, the next stop-signal delay was shortened by 50 ms, effectively making it easier to stop. These adjustments ensured that responses were inhibited successfully in about half of the stop trials, a requirement for estimating stop-signal reaction time that compensates for individual differences in choice reaction time to the go arrows.
26 SSRT was computed using the integration method described by Logan (1984).
25 Participants first completed a block of 60 practice trials, followed by five blocks of 60 experimental trials. Thus, 75 stop trials were obtained, which yields a reliable estimate of SSRT.
26Vocal Stop-Signal Task
The vocal version of the stop task required participants to orally name a series of pictured items (simple line drawings of familiar objects) quickly (e.g. say “tree” to the picture of a tree). The line drawings appeared in black ink against a light gray background and were framed within a square box measuring 7 cm×7 cm. The 60 pictured objects represented monosyllabic and bisyllabic words selected from a well-described set of pictures.
27 The objects depicted in the pictures were counterbalanced across several dimensions, including number of syllables, frequency of use, complexity, and naming reaction time. Before administration of the vocal stop task, participants viewed and named each pictured object aloud to ensure correct identification and name for each object. Vocal responses were registered using a microphone, with response time measured as the time from picture onset to the detection of vocal sound. The microphone voice detection settings were configured individually to achieve high voice detection fidelity. Upon naming the pictured object or a lapse of 1,200 ms without a naming response, the picture disappeared and an interval ranging between 1,000 and 1,500 ms transpired before the onset of the next picture. On 25% of trials, the frame around the box turned red shortly after the onset of the picture, and this color change instructed participants to stop their vocal naming of the pictured item. The stop-signal delay between the picture onset and the stop signal was set initially at 200 ms and then adjusted dynamically by 50 ms as described above for the manual stop-signal task. Naming responses were recorded during the experiment, and their accuracy decoded offline.
18 Participants first completed a block of 60 practice trials, followed by five blocks of 60 experimental trials. Inadvertent triggers of the voice microphone caused by nonverbal utterances or hesitation responses were excluded from analyses and accounted for an average of 5.1% of all vocal responses for each group.
Statistical Techniques
Extreme reaction time (RT) values, either excessively fast (so-called anticipatory errors; <150 ms) or slow (>3 standard deviations), were removed from the analysis. On average, 1.1% of trials were excluded per subject. For each task, three dependent measures were computed: go signal choice reaction time (GoRT), go signal accuracy (GoAcc), and stop-signal reaction time (SSRT). Additionally, the probability of successful inhibition on stop trials was computed to verify that the tracking algorithm in each task approximated the 50% stop success rate required to estimate SSRT.
25 A second measure, the mean RT for unsuccessfully inhibited responses on stop trials (i.e., failed stop trials), was computed for each task and compared with mean go RT to verify a key assumption of the stop task regarding the independence of the go and stop processes that is required to estimate stopping latency (SSRT) reliably
25; specifically, mean RT for unsuccessfully inhibited stop trials should be shorter than mean RT for go trials. These measures were submitted to separate overall mean analyses (analysis of variance; Huynh-Feldt adjustments for violations of sphericity) to verify the reliability of estimating SSRT and to determine group differences in GoRT, GoAcc, and SSRT.
Results
Clinical Characterization
Participant demographic characteristics are presented in
Table 1. Compared with the HC group, the TS group endorsed higher ratings of anxiety (F[1,50]=13.96, p<0.001), depression (F[1,50]=6.72, p<0.05), attention-deficit/hyperactivity (F[1,50]=8.66, p<0.01), and obsessive-compulsive symptoms (F[1,50]=18.48, p<0.001). Importantly, the scores for the TS group fell in subclinical ranges.
Influence of TS on Manual Stopping Control
Final sample.
One participant from each group showed outlier stop-signal tracking results, rendering estimate of SSRT uninterpretable. Thus, the final sample for the manual stop-signal task consisted of 25 participants in each group. Mean performance values are presented in
Table 1.
Choice RT and accuracy.
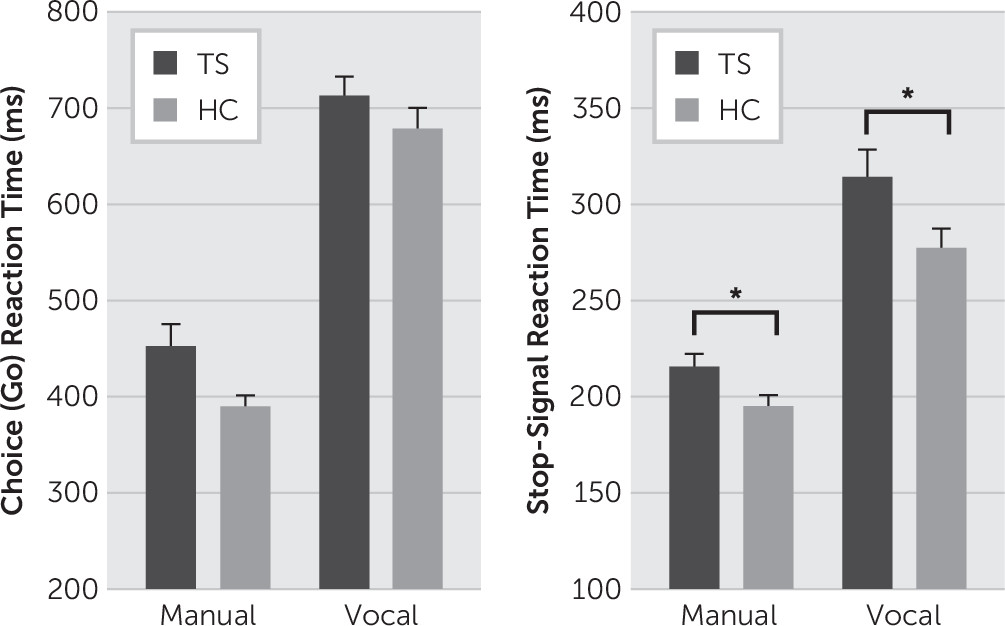
Compared with the HC group, the TS group tended to respond more slowly on go trials, (F[1,48]=4.00, p=0.051), yet performed with similar levels of high accuracy (TS=98.7%, HC=99.2%; F[1,48]=1.99, p=0.148) (
Figure 1A).
Manual stop-signal RT (mSSRT).
Stopping accuracy was similar and near 50% in both groups (TS=53.9%, HC=53%; group, F[1,48]=0.245, p=0.623). Mean RT for failed stop trials was shorter than mean RT for go trials (trial type, F[1,48]=79.06, p<0.001). This pattern was similar for TS (49 ms shorter) and HC (40 ms shorter) groups (group-by-trial type, F[1,48]=0.813, p=0.372). These analyses confirm the success of the tracking algorithm and the reliability of the estimate of manual stopping latency (mSSRT). As predicted, the TS group was significantly slower at stopping initiated manual actions compared with the HC group (TS=215 ms, HC=194 ms; F[1,48]=4.69, p=0.017, one-sided) (
Figure 1B).
Influence of TS on Vocal Stopping Control
Final sample.
Of the 26 original TS participants, eight participants had a history of vocal tics that remitted with age and were not present at the time of testing. These patients were excluded from the analysis of vocal stop-signal performance. The remaining group of 18 TS participants was rematched and compared with a group of 18 HC participants based on age and gender. Mean performance values are presented in
Table 1.
Choice RT and accuracy.
The TS and HC groups showed similar naming RTs (TS=717 ms, HC=677 ms) and accuracies (TS=99.6%, HC=99.9%; RT: F[1,34]=1.69, p=0.20; accuracy: F[1,34]=1.47, p=0.23) (
Figure 1A).
Vocal stop-signal RT (vSSRT).
Stopping accuracy was similar and near 50% in the TS group (51%) and the HC group (52.4%). Mean RTs for failed stop trials were shorter than mean RTs for go trials (trial type: F[1,34]=77.27, p<0.001), a pattern that was preserved across groups (TS: 84 ms shorter; HC: 60 ms shorter; group-by-trial type, F[1,34]=2.213, p=0.146). These analyses confirm the success of the tracking algorithm and the reliability of the estimate of vocal stopping latency (vSSRT). Consistent with the pattern measured for manual stopping, TS patients were significantly slower at stopping initiated vocal actions compared with the HC group (TS=313 ms, HC=276 ms; F[1,34]=3.62, p=0.03, one-sided) (
Figure 1B).
Returning to the eight patients who had persistent motor tics and a history of vocal tics that had dissipated with age, we compared manual and vocal SSRTs between these patients and the 17 TS patients who had both motor and vocal tics at testing (recall that one patient had uninterpretable manual SSRT data). Interestingly, the eight patients presenting with motor but not vocal tics showed prolonged and indistinguishable manual SSRTs from the TS patients presenting with both vocal and motor tics (213 versus 216 ms) (t[23]=0.18, p=0.861). However, vocal SSRTs among the same eight patients (248 ms) were significantly shorter than the group presenting with both types of tics (313 ms), (t[23]=2.25, p=0.035). This raises the possibility that deficient inhibitory control over manual and vocal actions may track with the presence or remission of manual and vocal tics.
Association of Performance Variables to Clinical Features and Treatment of TS
Manual SSRT did not correlate with clinical ratings of tic severity (i.e., total YGTSS tic severity score) (r=−0.103, p=0.624), ratings of ADHD (r=0.396, p=0.050), OCD (r=−0.017, p=0.935), anxiety (r=0.105, p=0.616), and depression (r=0.039, p=0.852). Similarly, vocal SSRTs did not correlate with clinical ratings of tic severity (i.e., total YGTSS tic severity score) (r=0.056, p=0.824), ratings of ADHD (r=−0.005, p=0.983), OCD (r=0.242, p=0.333), anxiety (r=−0.085, p=0.739), and depression (r=−0.319, p=0.198). TS patients taking versus not taking tic medications did not show differential overall reaction time (GoRT) or stopping speed (i.e., SSRT) for manual (RT: F[1,23]=0.036, p=0.852; mSSRT: F[1,23]=1.19, p=0.29) and for vocal (RT: F[1,16]=0.099, p=0.757; vSSRT: F[1,16]=0.196, p=0.66) responses. In a separate subgroup analysis, there were no differences between TS patients taking SSRI/tetracyclic medications versus those not taking these medications on mean manual GoRT, mSSRT, vocal GoRT, or vSSRT (all p values >0.10).
Discussion
We tested the hypothesis that late adolescents and young adults with relatively uncomplicated, persistent TS are slower to inhibit voluntarily initiated manual and vocal actions. While the TS group showed intact speed to execute both manual and vocal actions, they were decisively slower than HCs in their effort to stop these action processes once they were initiated. To our knowledge, this is the first report of slowed inhibition of vocal reactions in TS.
Studies are suggestive that efforts to suppress tic behavior among children and young adolescents with TS may foster development of compensatory neural circuitry that enables more proficient inhibitory control capabilities.
7,9,28 However, the current findings suggest that unremitting TS into late adolescence or adulthood may express a specific vulnerability in inhibitory control circuitries.
10,29,30 Imaging data has also linked persistent TS in early adulthood to structural and functional differences in prefrontal and basal ganglia circuitries that are implicated in inhibitory control. A particularly intriguing finding in the current study was demonstration that a small subset of TS patients with persistent motor but remitted vocal tics showed delayed manual SSRTs similar to TS patients with persistent motor and vocal tics but markedly shorter vocal SSRTs. This accords with the idea that the expression of manual or vocal inhibitory control deficits is linked with the persistence or remission of tic expression in a particular effector system. However, the cross-sectional design used in this and past studies is nondisclosing about whether tics in some patients might have remitted in the future. This limitation could be addressed by longitudinal designs that dissociate the evolution of inhibitory control capabilities in individuals with TS whose symptoms remit versus persist into adulthood.
Slower manual stopping speed accords with a past study of adults with TS that excluded patients with comorbid diagnoses of OCD or ADHD.
15 Certainly a broader application to individuals with TS is a
limitation given the high rates of neuropsychiatric comorbidities in the general TS population. We made efforts to mitigate the likely impact of these comorbidities by screening for them prospectively. However, we acknowledge important limitations of using self-report ratings and routine clinical interviews in the detection of a past history of psychiatric comorbidities. Even though TS participants denied a past diagnosis of ADHD or OCD and did not meet current criteria for major mood disorder, they showed higher subclinical tendencies on rating scales compared with the HC group. Thus, we cannot entirely rule out the potential influence of milder, subclinical manifestations of psychiatric comorbidities. The absence of correlations between ratings of psychiatric comorbidities and manual or vocal stopping latencies mitigates to some extent concerns that these mild elevations influenced stopping control. However, because these comorbidities are also associated with inhibitory control deficits, a key question for future research is how these conditions interact to influence cognitive control processes and confer additional risk for long-term inhibitory control deficits.
31,32Vocal and manual stopping have been associated with similar neural mechanisms.
33 In a study of healthy adults performing manual and vocal versions of the stop task, Xue et al.
34 reported that the
initiation of manual and vocal actions engaged dissociable motor areas (e.g., primary motor cortex for manual; left prefrontal cortex for speech production areas), but that the
inhibition of vocal and manual actions similarly activated right inferior frontal cortex and presupplementary motor area. These cortical regions, along with their connections to the basal ganglia, comprise the brain’s inhibitory control network, a network that is variably impacted in adults with persistent TS.
10,35 Moreover, Ganos et al.
36 identified functional changes in this network between TS and controls performing a stop-signal task.
It should be recognized that several measures of inhibitory control used in the TS literature are not entirely interchangeable, but capture distinct forms of inhibitory control associated with dissociable neural mechanisms. For instance, a handful of investigations of children and adults with TS have measured inhibitory control over prepotent reactions using a disjunctive reaction paradigm (i.e., Go/No-Go task) and generally reported the absence of differences in commission errors compared with HCs.
37–39 This paradigm measures restraint from initiating prepotent action tendencies, a form of preparatory (or proactive) inhibitory motor control that is distinctly different from the reactive inhibitory process engaged to stop an intentionally initiated action as measured by the stop-signal task.
40,41 Using a response conflict task, we showed that adults with relatively uncomplicated, persistent TS were no more susceptible to acting on strong motor impulses but were less proficient at inhibiting interference from these impulses compared with healthy peers.
16 In light of these findings, a more precise conclusion is that TS does not appear to impact preparatory (or proactive) control processes critical to restraining impulsive actions, but it may alter inhibitory control processes engaged reactively to suppress already activated but unwanted motor plans.
From a clinical perspective, recognition that persons with TS, including young adults with persistent TS symptoms, show deficits in inhibitory motor control mechanisms is important for developing targeted therapies and a more complete understanding of the clinical heterogeneity of TS. Whether alterations in compensatory and deficient inhibitory control reflect specific adaptations or vulnerabilities in the structure and maturation of frontal-basal ganglia circuitries remains an open empirical question for future research.
Acknowledgments
The authors thank Bert van Beek for programming the computer task. The authors also thank Ms. Laura Wegner for study coordination and Dr. G. Frederick Wooten for assistance in participant recruitment.