Poststroke depression has been increasingly recognized as one of the most important consequences of stroke, as depression is common and often recurrent,
1–3 associated with poor outcomes,
4 costly,
5,6 and difficult to treat given currently available treatments.
7 The impact of poststroke depression is perhaps most pronounced as a barrier to recovery of functional status,
8 independence,
9 and long term survival after hospital discharge.
10–12 Although antidepressants are now more frequently prescribed after stroke, there has been no clear decrease in the prevalence of poststroke depression.
2,7 Because the effectiveness of antidepressants and other interventions treating and resolving poststroke depression appear limited, prevention has gained much more interest. Accumulating evidence suggests that long term reductions in depressive symptoms are best achieved through targeting individuals
prior to the onset of their symptoms, rather than
after the disorder emerges.
13 However, to determine whether early treatments such as antidepressants can prevent stroke-related depression, it is critical to predict which patients are most likely to develop poststroke depression at the time of stroke. We undertook this study to better identify these patients of higher susceptibility.
In order to prevent cases of poststroke depression by identifying patients
before the onset of depression, there is a need to understand
new-onset depression poststroke and the prestroke risk factors that predict its development. New-onset depression poststroke is a distinct and important case of poststroke depression, as it may help clarify the influence of risk factors for depression unique to stroke. Using the combination of prestroke factors and cases of new-onset depression poststroke may help identify those cases where an early empiric antidepressant after stroke or a behavioral intervention may prevent the development of poststroke depression.
14 Candidate risk and protective factors linked to poststroke depression have been proposed, though extant studies are limited in three primary ways. First, prior studies are typically confined to clinical and convenience samples where information about prestroke factors is often limited. Second, previous research has largely focused on poststroke predictors [for examples, see
1,4] rather than on risk factors present prior to the stroke. Third, studies examining prestroke measures (including depressive symptoms) do so in a retrospective manner, which is less reliable than prospective ascertainment. Thus, to better identify stroke survivors at risk for depression who may benefit from a targeted prevention strategy in the acute to subacute poststroke period, we examined whether prestroke factors were associated with the risk for developing new-onset depression poststroke.
Results
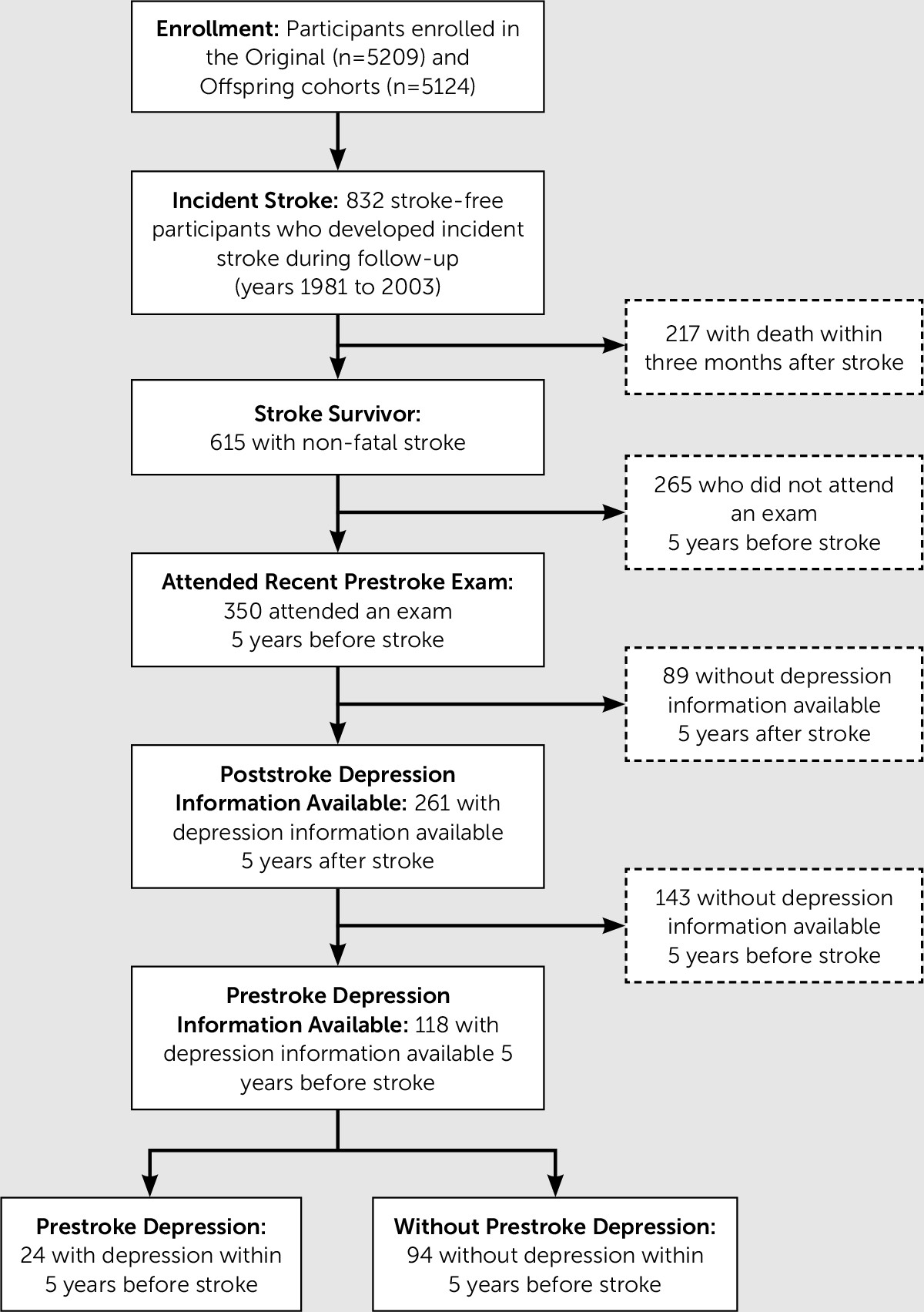
Among the participants for whom prestroke assessment of depressive symptoms was available within 5 years before and after stroke, 94/118 (80%) did not have recent history of depressive symptoms before stroke. The mean (SD) times from prestroke CES-D assessment to stroke and from stroke to poststroke CES-D were 2.1 (1.4) years and 1.9 (1.3) years, respectively. The mean (SD) times from prestroke medication inventory to stroke and from stroke to poststroke medication inventory were 1.9 (1.5) years and 2.2 (1.4) years, respectively. There were no significant differences between participants who did not have depression information available within 5 years after stroke compared with those that did. Poststroke depression of any type (i.e. including those with previous history of depression) was present in 49/118 (42%) where 11 were on antidepressant medication and had CES-D≥16, 28 were not on antidepressant medication but had a CES-D≥16, and 10 were on antidepressant medication and had CES-D<16. Of these cases, a majority (34/49, 69%) did not have recent history of depressive symptoms 5 years prior to stroke and thus were classified as having new-onset depression poststroke. Among the 34 participants with new-onset poststroke depression, nine were on antidepressant medication and had CES-D≥16, 18 were not on antidepressant medication but had CES-D≥16, and seven were on antidepressant medication and had CES-D<16). At-risk participants in the primary analytic sample who developed new-onset depression after stroke (34/94, 36%), compared with those who did not, were more likely to be older, women, current smokers, and dependent in activities of daily living (
Table 1).
The odds of developing new-onset depression poststroke was 1.08 greater for each 1-year difference in age at the time of stroke (95% CI, 1.02–1.15; df=1; p=.009) (
Table 2). Controlling for age only, women had a 3.30-fold increase in the odds of experiencing new-onset depression poststroke compared with men (95% CI, 1.30–8.37; df=2; p=.012). After controlling for age, sex, and time from prestroke assessment to date of incident stroke, we found that smokers had 8.87 times the odds of developing new-onset depression poststroke compared with nonsmokers (95% CI, 1.19–66.11; df=3; p=.033) and dependent bathing prior to stroke onset had an OR of 4.65 (95% CI, 1.00–21.69; df=3; p=.050).
In post hoc sensitivity analyses, we defined new-onset poststroke depression cases solely by CES-D≥16 (i.e. the seven participants that were on antidepressant medication and had CES-D<16 were no longer classified as cases). Advanced age (OR 1.15; 95% CI, 1.06–1.24; df=3; p=.001) and prestroke smoking (OR 13.95; 95% CI, 1.60–121.39; df=3; p=.017) remained associated. Odds of developing new-onset depression poststroke was 1.15 greater for each one-point difference in BMI (95% CI, 1.01–1.31; df=3; p=.041) (
Table 3). We also examined the association between sex and new-onset poststroke depression in one model additionally adjusted for age and smoking and a second model additionally adjusted for age and dependent bathing. Adjustment for smoking had a minor impact on effect with a change in OR from 3.30 (95% CI, 1.30–8.37; df=4; p=.012) to 2.99 (95% CI, 1.10–8.05; df=4; p=.032). Adjustment for dependent bathing, reduced the OR to 2.36 (95% CI, 0.84–6.65; p=.104). The overall effect of dependent bathing in women (N=48) was a 9.82-fold increase in the odds of developing new-onset poststroke depression (95% CI, 1.24–77.51; df=4; p=.030).
Discussion
Our study demonstrated that among those who developed poststroke depression, the majority did not have depression 5 years prior to their stroke (34/49, 69%); however, the risk of poststroke depression is still higher in those with prestroke depression (15/24, 63%) than in those with no prestroke depression (34/94, 36%). We found that women, older adults, smokers, and those with some dependence in activities of daily living at the time of stroke were more likely to develop a first episode of depression poststroke. The results of post hoc sensitivity analyses add confidence to identified associations with advanced age and smoking and suggest that women may be more likely to become depressed poststroke if they have functional impairment before the stroke.
Prior studies in mostly hospital- and rehabilitation-based registries have identified advanced age
3,4 at the time of stroke as associated with poststroke depression. Consistent with the established association between women and risk of depression, our findings suggest women are at higher odds of new-onset depression poststroke. This finding, however, is at odds with prior studies of poststroke depression, which have shown either no differences in risk by sex, or higher risk among men.
25,26 Moreover, the greater odds of new-onset depression poststroke among smokers may be explained by the proposed pathophysiologic models for poststroke depression from previous associations implicating chronic inflammatory cytokines and poststroke neuroinflammatory response
27 as well as impaired endothelial function
28 and changes in vascular perfusion.
22 This finding differs from one previous study that did not detect an association with smoking status
29 though may be supported by the association with obesity in post hoc sensitivity analysis. The absence of associations with cardiovascular disease, hypertension, and diabetes mellitus may be related to either small effect sizes or a more direct pathophysiologic role of white matter microstructural damage, which has been more specifically associated with apathy as a contributing factor in the development of poststroke depression.
30 The association with poststroke impairments in activities of daily living has been demonstrated,
1 though our results indicate that prestroke functional status may also be an important factor to consider as it implies a potential relationship between greater cumulative years of functional disability and new-onset depression poststroke.
In our prospective study of participants followed at least 5 years prior to their stroke, the occurrence of poststroke depression and new-onset depression poststroke (42% and 29%, respectively) parallels previous poststroke depression estimates of about 33% in the first 3 months after stroke and suggests that the actual incidence of new-onset depression poststroke is greater than 30%.
1,2,7 Clinical and convenience samples have reported more disparate incidence and prevalence estimates for poststroke depression. However, while the burden of poststroke depression has wide variation in its estimates, precise estimates of new-onset depression poststroke are even more challenging to discern.
6,31 We suspect wider estimates from other studies may reflect selection bias (by including only the most disabled or least disabled stroke survivors), particularly in rehabilitation-based studies.
32 This is likely due to inherent clinical challenges in detecting poststroke depression, known classification challenges in measuring psychiatric symptoms using retrospective reports, and the ability to distinguish new-onset depression poststroke from relapse of longstanding major depressive disorder.
5 Our study overcomes this limitation by using a prospective cohort with minimal loss to follow-up, which also made it possible to uncover that 69% of poststroke depression occurred in those
without a recent history of depressive symptoms before stroke.
Strengths of this study include its longitudinal design, large incident stroke sample, and the close surveillance of clinical end points with minimal loss to follow-up. Results, however, need to be interpreted in the context of limitations. First, we were unable to account for the potential effects of additional factors, such as family history of depression or stroke heterogeneity in size, etiology, and neuroanatomical involvement. Second, generalizability of results may be limited, given that these analyses were conducted in a sample of largely European ancestry. Third, an even larger study of stroke survivors who were free from stroke and depression at baseline may generate more precise effect estimates, especially when there is potential for nondifferential misclassification from a tendency to underdiagnose poststroke depression.
5 The FHS mitigates some of these effects with its frequency of assessments.