Cognitive impairment occurs in a significant proportion of patients with temporal lobe epilepsy (TLE).
1,2 Although the most prominent cognitive deficit in TLE is memory impairment, secondary to the effects of the primary temporal lobe epileptogenic lesion,
3 more recent studies demonstrate diffuse cognitive impairment.
4 One area of cognition that is increasingly studied in TLE is executive functioning. Executive functions refer to higher-order cognitive processing involved in goal-directed behaviors such as initiation, self-monitoring and regulation, planning, organization, inhibition, and abstraction.
5 Among patients with TLE, executive deficits include reduced problem solving,
6–9 mental control/working memory,
10,11 cognitive flexibility,
9,12 verbal fluency,
9,13 inhibition,
10,14 and decision making.
15 Detection of executive dysfunction in TLE is of particular importance given its strong association with functional impairment and disability.
16Numerous factors contribute to executive dysfunction in TLE, such as duration of epilepsy, age of epilepsy onset, seizure type, and seizure lateralization/localization, with the majority of studies demonstrating more severe effects with earlier onset and left-sided seizure focus.
7,10,11 Neuroimaging studies have helped to clarify the neurobiological basis of executive dysfunction in TLE, with several implicating the frontal lobes
12,14,17 and others highlighting the role of the hippocampus.
9,15,18,19 Using FDG-PET, Jokeit and colleagues found that asymmetric prefrontal hypometabolism was associated with executive dysfunction.
17 In a more recent study, poorer performance on the Wisconsin Card Sorting Test (WCST), a measure of problem solving, was associated with glucose hypometabolism in the prefrontal cortex in TLE among patients with frequent seizures.
12 Using MRI, Keller and colleagues demonstrated that higher working memory scores were related to greater volume in the left and right dorsal prefrontal cortex (DPFC) and ventral prefrontal cortex (VPFC) but not to hippocampal volume, while better verbal fluency was associated with greater left hippocampal and DPFC volume. This study also demonstrated that better response inhibition (Stroop test) was associated with greater left VPFC volume.
14 Other studies highlight the role of the hippocampus, showing that hippocampal sclerosis is associated with executive dysfunction in TLE. For example, Corcoran and Upton demonstrated that patients with hippocampal sclerosis made more perseverative errors and completed fewer categories on the modified WCST compared with TLE patients without hippocampal sclerosis.
18 These findings are consistent with other studies demonstrating a contribution of hippocampal sclerosis to executive dysfunction using the WCST
9,19 and the Iowa Gambling Task.
15 Most recently, a study using diffusion tensor imaging implicated connectivity between the frontal and temporal lobes in executive deficits in TLE. Specifically, among TLE patients, reduced integrity of the left uncinate fasciculus was related to poorer performance on tasks of basic attention and word retrieval (verbal fluency and digit span).
13Another likely contributor to executive dysfunction in TLE is depression. Depression is very common in TLE, affecting 25%−55% of patients,
20,21 and there is a well-established relationship between depression and executive dysfunction among nonepileptic adults.
22,23 Converging evidence suggests a neurobiological basis for this relationship,
24–36 including involvement of overlapping brain structures, namely, the hippocampus
27–29 and frontal lobe structures.
30–33 Additionally, there is a well-established relationship between depression and executive dysfunction in other populations.
22,23 However, the contribution of depression to executive deficits in TLE has not been widely studied. In one study, greater perseverative responses on a novel problem-solving task were associated with higher levels of depressive symptoms among individuals with left TLE.
6 In another study, patients with TLE with comorbid depression performed significantly poorer than nondepressed patients with TLE on numerous cognitive measures, including tasks of executive functions, after controlling for seizure frequency.
34 These studies, however, did not have a comparison group of lone depression.
The extent to which depressive symptoms add to executive deficits in TLE remains unclear. To address this question, the present study examined the contribution of depression to executive functioning in TLE using three well-matched groups: nonneurologic patients with depression, patients with TLE and depression, and nondepressed patients with TLE. A battery of executive function tests was used to more broadly investigate the nature of executive dysfunction in TLE and depression. It was hypothesized that patients with TLE and comorbid depression would show greater executive dysfunction on a series of executive functioning tasks than would nondepressed patients with TLE or neurologically healthy depressed patients. It was also hypothesized that among TLE patients, greater depressive symptoms would be associated with greater executive deficits.
Methods
Participants
Eighty-two adults participated in the current prospective study. They were of three groups: TLE without depression (TLE; N=29), TLE with depression (TLE+DEP; N=22), and depression without TLE (DEP; N=31). Group membership was determined by responses on the Mini International Neuropsychiatric Interview, a semistructured interview for psychiatric diagnoses.
35 Individuals included in both the DEP and TLE+DEP groups were required to meet criteria for major depressive disorder or dysthymic disorder. Criteria correspond to
DSM-IV.
36 All patients spoke English as their primary language. Patients with TLE were recruited from an outpatient neurology practice and were required to have complex partial epileptic seizures of definite or probable temporal origin as defined by continuous video-EEG monitoring of spontaneous seizures demonstrating temporal lobe seizure onset. Three participants had MRI evidence of hippocampal sclerosis. There were no other participants with a structural epileptogenic lesion or vagal nerve stimulator. The depression group was recruited from hospital-based outpatient psychiatry clinics and community advertisements. Exclusion criteria for both DEP and TLE groups were 1) other neurologic condition unrelated to the etiology of the seizure disorder (e.g., multiple sclerosis, Parkinson’s disease, stroke, dementia); 2) history of intellectual disability; 3) history of or current substance abuse (
DSM-IV criteria); and 4) history of bipolar disorder or psychosis (
DSM-IV criteria).
Measures
The 17-item Hamilton Depression Rating Scale (HAM-D)
37 is a 17-item, clinician-rated interview that measures syndromal depression on a Likert scale. The HAM-D was used as measure of current depression symptom severity. The dependent variable was total score, with higher scores indicating greater depressive symptomatology. The present study used the continuous HAM-D score in analyses. Scores are also classified as follows: 0–7=normal, 8–13=mild depression, 14–18=moderate depression, 19–22=severe depression, and 23+=very severe depression.
The two-subtest form of the Wechsler Abbreviated Scale of Intelligence
38 was administered to obtain a brief measure of intelligence to ensure that groups were matched on global cognitive skills.
The Delis-Kaplan Executive Function System (D-KEFS)
39 is comprised of nine subtests that measure a spectrum of verbal and nonverbal executive skills. Seven of the subtests were used in this study to measure a range of executive skills, including the following:
Trail-Making. This test is an adaptation of the traditional Trail-Making Test
40 and is used to assess sequencing, motor speed, and cognitive flexibility using five conditions: 1) visual scanning in which participants cross out all of a specified letter presented among various other letters as quickly as possible, 2) letter sequencing in which participants connect letters in alphabetical order as quickly as possible, 3) number sequencing in which participants connect numbers in numerical order as quickly as possible, 4) number-letter switching in which participants must alternate between letters and numbers in alphabetical and numerical order as quickly as possible, and 5) motor speed in which participants trace over a dotted line connecting empty circles as quickly as possible. The five dependent variables used were completion times for each of these conditions.
Verbal fluency. This test assesses the ability to generate words as quickly as possible across three conditions: 1) letter fluency in which the participant is asked to say as many words as possible starting with a specified letter, 2) category fluency in which the participant asked to say as many words as possible that belong to a specified semantic category, and 3) category switching in which the participant must alternate between saying words from two different semantic categories. The three dependent variables used from this subtest were total correct from each condition.
Design fluency. This subtest measures visual productivity and cognitive flexibility using conditions with and without distracting stimuli. Participants are asked to produce as many designs as possible in three conditions: 1) filled dots, 2) empty dots, and 3) switching between empty and filled dots. The three dependent variables used from this subtest were total correct for each of the conditions.
Color-Word Interference. This test assesses processing speed, inhibition, and cognitive flexibility in four conditions: 1) word reading in which participants read a list of color words (i.e., red, green, blue) as quickly as possible, 2) color naming in which participants states the color of a series of colored rectangles (i.e., red, green, blue) as quickly as possible, 3) inhibition in which participants are asked to name the color of ink that an incongruent color word is printed in (i.e., say “blue” when the word “red” is printed in blue ink), and 4) inhibition switching in which participants have to switch back and forth between naming the incongruent ink color and reading the printed word. The four dependent variables for this subtest are the completion times for each of the conditions.
Sorting. This is a test of problem-solving and concept formation that contains two conditions: 1) the free- sorting condition requires participants to sort six cards containing a word and a picture on the basis of as many rules as possible and 2) sort recognition in which the cards are sorted and the participant must identify the rules by which the cards are sorted. The three dependent variables are the number of correct sorts for the free-sorting condition and the description of the sorting for the free and recognition conditions.
Word context. This is a test of deductive reasoning/abstraction in which participants are provided with a fictitious word and must determine the word’s meaning through provided clues. The dependent variable is correct responses.
Proverb test. This is a test of verbal abstraction that has two conditions: 1) the free condition in which proverbs are read and the participant is asked to interpret and 2) the multiple-choice condition in which participants are read a proverb and chooses the best interpretation from four options. The two dependent variables from this subtest are the total achievement scores for each condition.
Procedure
Patients meeting criteria for inclusion were scheduled for an in-office appointment and provided informed consent at the onset of their visit. Neuropsychological tests were administered by trained research staff. Mood assessments were administered by a neuropsychologist who was blind to the participant’s cognitive performance. Chart review was conducted to obtain demographic variables and epilepsy characteristics. All procedures were approved by the local institutional review
Data Analysis
Scores for the D-KEFS subtests were corrected for age using normative data from the test manual and converted to scaled scores (mean of 10, standard deviation of 3; higher scores reflect better performance) to facilitate interpretation. Independent-samples t test and chi-square analyses were used to examine group differences in demographic and clinical characteristics. One-way analysis of variance (ANOVA) was used to examine differences between groups on D-KEFS scores. Post hoc analyses using Bonferroni adjustment for multiple comparisons were used to clarify significant results. Subtests were not grouped into composite scores because D-KEFS tasks represent a diverse set of executive abilities. Bivariate correlations were then used to examine the association between depressive symptoms from the HAM-D and performance on each of the individual D-KEFS scores in the total TLE group (TLE and TLE+DEP) and the DEP group. Follow-up hierarchical linear regressions were conducted to further examine the significant associations between depression and test performance for the TLE group. For each regression, seizure frequency, years living with epilepsy, and antiepileptic drug (AED) mono/polytherapy were added in block 1, and depressive symptoms from the HAM-D were added in block 2. Significance was defined as p<0.05.
Results
Demographic and Clinical Characteristics
Table 1 presents the demographic and clinical characteristics of the groups. There were no between-group differences for age, education, estimated IQ, or ethnicity. There were differences in gender between groups such that there were fewer females in the TLE group compared with the other two groups. For the TLE groups, three had MRI findings of hippocampal sclerosis. All other TLE participants had nonstructural epileptogenic foci. No participants had vagal nerve stimulators. Depressed groups were matched on depression severity as measured by the HAM-D, with symptoms falling in the mild range. Both groups also had a similar proportion of participants meeting criteria for major depressive disorder versus dysthymic disorder.
Associations
AED polytherapy was related to poorer performance on letter fluency (t[48]=2.87, p<0.01), design fluency filled dots (t[48]=1.13, p=0.04), and word context consecutively correct (t[45.40]=3.36, p<0.01). There were no other significant correlations between test performance and poly- versus monotherapy. Longer duration of epilepsy was associated with better performance for color naming (r=0.54, p<0.001) and word reading (r=0.32, p=0.03). There were no other significant correlations between epilepsy duration and test performance. There were no significant differences between seizure frequency groups on test performance for any of the measures. There were also no differences between genders on any of the cognitive tests. Given the significant associations, AED poly/monotherapy and epilepsy duration were included as covariates in later analyses.
Group Differences in Neuropsychological Test Performance
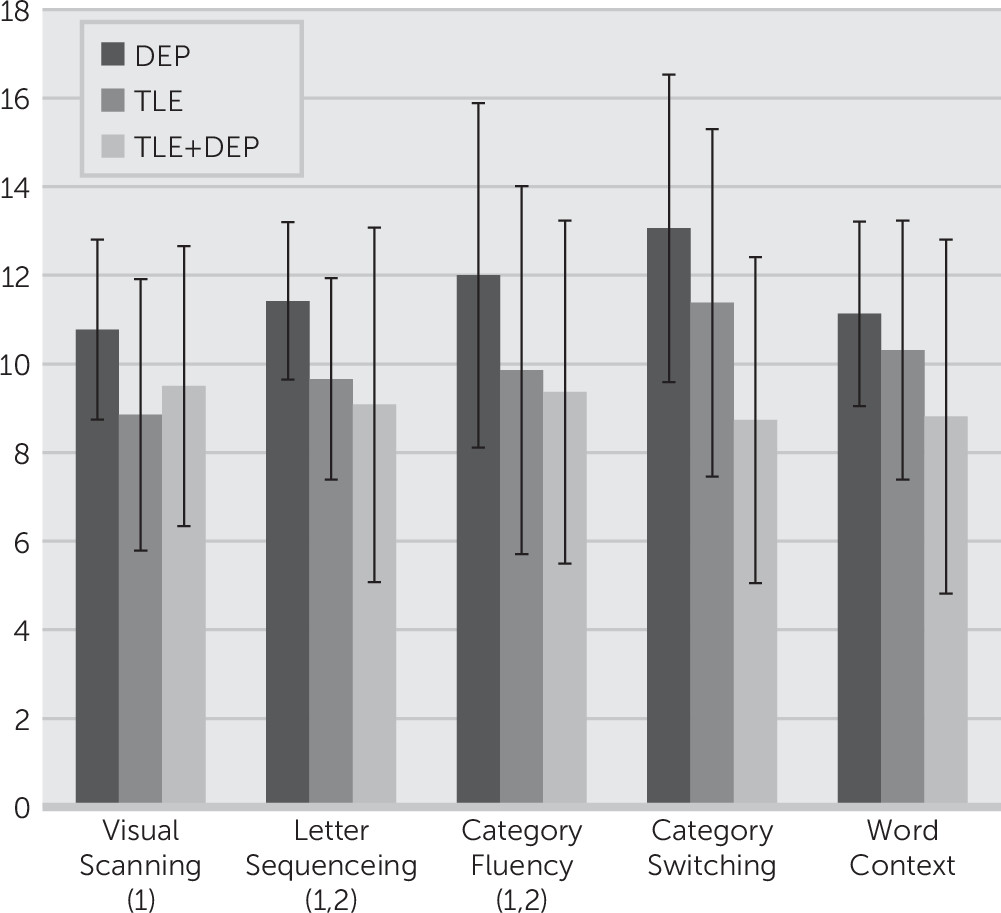
One-way ANOVAs were conducted to examine group differences (TLE versus TLE+DEP versus DEP) on each of the D-KEFS scores. Results demonstrated significant overall group differences for visual scanning, letter sequencing, category fluency, category switching, and word context consecutively correct. Post hoc analyses indicated that the DEP group performed better than the TLE group for visual scanning (p=0.02) and letter sequencing (p=0.04). The DEP group performed better than the TLE+DEP group for letter sequencing (p<0.001), category switching (p<0.001), word reading (p=0.04), and word context consecutively correct (p=0.03). The TLE group performed better than the TLE+DEP group for category switching (p=0.04). Full results of the group differences are presented in
Table 2.
Figure 1 presents scores for subtests on the D-KEFS for which there are overall significant between-groups differences.
Depressive Symptoms are Associated With Executive Functions
Bivariate correlations were conducted to examine associations among scores on the D-KEFS and HAM-D-17 separately for the depression and the combined TLE group (TLE, TLE+DEP) to explore the impact of depression on cognition in neurologically healthy versus participants with TLE. In the TLE group, there were significant correlations indicating poorer performance with higher depressive symptoms for category switching on the verbal fluency subtest (r=−0.39, p<0.01), word reading (r=−0.33, p=0.02), and word context total consecutively correct (r=−0.29, p=0.04). For the depression group, greater depressive symptoms were associated with slower motor speed (r=0.43, p=0.02) and lower scores for design fluency filled dots (r=−0.38, p=0.04).
Hierarchical linear regressions were used to examine whether depressive symptoms were related to executive functions among the combined TLE group (TLE, TLE+DEP) after controlling for seizure characteristics including years living with epilepsy and AED monotherapy versus polytherapy (coded 0, 1, respectively). Of note, one participant was not on any AEDs and was therefore excluded from these analyses. Regression coefficients for variables in the final block are presented in
Table 3.
For verbal fluency category switching, the first block including epilepsy duration, and AED monotherapy/polytherapy was not statistically significant (F[2, 47]=1.11, p=0.34). The second block, which included the HAM-D, was statistically significant (F[3, 44]=3.17, p=0.03; ∆F=7.00, p=0.01) and accounted for 17.1% of variance. In the final model, only higher HAM-D scores were related to worse category-switching performance. For word reading, the first block including years with epilepsy, and AED monotherapy/polytherapy was not significant (F[2, 46]=2.67, p=0.08). The second block, which included the HAM-D, was significant (F[3, 45]=5.35, p<0.01; ∆F=9.70, p<0.01) and accounted for 26.3% of variance. In the final model, longer epilepsy duration and lower HAM-D scores were related to better word-reading performance.
For word context score, the first block, including years living with epilepsy and AED monotherapy/polytherapy, was significant (F[2, 47]=4.83, p=0.01). The second block, which included the HAM-D, was also significant (F[3, 46]=4.28, p=0.01) and accounted for 21.8% of the variance. Only AED monotherapy/polytherapy was a significant predictor in the final model.
Discussion
The present study examined the unique contributions of TLE and depression on executive functioning in three separate cohorts of patients with TLE, comorbid TLE and depression, and depression alone. Findings first replicated prior studies showing that patients with TLE exhibit poorer executive functioning relative to individuals without TLE.
6–8,10,12,13 Neuroimaging studies support prefrontal hypometabolism and reduced prefrontal cortical volume in patients with TLE, suggesting that structural and functional extratemporal abnormalities underlie executive dysfunction in TLE.
12,14The second goal of this study was to examine the potential cumulative effect of depression on executive functioning by directly comparing executive functioning in TLE groups with and without depression and to a neurologically healthy group with depression alone. The majority of findings showed differences between the TLE patients with comorbid depression versus depression alone. Specifically, TLE patients with depression showed significantly worse performance on several aspects of executive functioning related to speed, verbal abstraction, and verbal mental flexibility compared with those with depression alone. The effect was less robust when examining the unique contribution of depression to executive functioning in the TLE groups. The TLE versus TLE+DEP groups differed only on tasks of verbal mental flexibility.
It was hypothesized that greater depressive symptoms, as measured by a state measure of mood, would be associated with greater executive deficits in TLE. This was supported to a modest degree. Greater depressive symptoms were related to poorer performance on tests of cognitive flexibility, processing speed, and deductive reasoning among the TLE participants as a whole. Importantly, these relationships persisted after controlling for epilepsy characteristics including duration of epilepsy, seizure frequency, and AEDs. Interestingly, bivariate correlations between executive functions and depressive symptoms in the depression we associated with different aspects of executive functioning than within the TLE group, namely, speeded tasks (i.e., motor speed and design fluency). This is consistent with prior literature showing that depression impacts processing speed.
41 It was somewhat surprising that the relationship between depression and executive functioning was different in TLE versus depression alone. In the TLE group, depression seemed to affect verbally mediated tasks of executive functioning, whereas depressive symptoms in the depression-only group were related to pure speed and visually mediated executive functions. Future studies may wish to examine the impact of depression on executive function in right versus left TLE. Our sample size does not allow for further investigation of lateralization.
The present study must be viewed in light of several limitations. First, the cross-sectional nature of the data precludes causal interpretations. Longitudinal designs could be used in future studies to examine whether improvements in depression through treatment can result in improved executive skills, which has been shown in other neurological populations
42 and neurologically healthy depressed individuals.
43 Second, this sample was comprised of largely cognitively intact individuals, as demonstrated by mean scores on the executive function tests falling within the average range compared with same-age peers. Whether these findings are present in lower-functioning samples with epilepsy was not addressed in this study. This sample was comprised of patients with well-controlled epilepsy, as demonstrated by average seizure frequency of one or fewer seizures per month. As in prior studies, epilepsy severity indices were not consistently associated with cognitive functioning. The significance of the finding that longer seizure duration was associated with faster processing speed is unclear. Seizure duration was simply years since diagnosis of epilepsy and did not factor in seizure control over the years. As such, this variable may not be a sensitive proxy for disease severity and may have produced a spurious relationship with this one aspect of cognition. Additionally, small sample size limited our ability to examine the possibility that the relationship between depression and executive function in TLE differs by epilepsy lateralization. Previous research has demonstrated that the contribution of depression to executive deficits may depend on the side of seizure focus.
6 There was a greater percentage of females within the depression groups, which may impact the applicability of these findings to men, but there were no differences between men and women on any of the cognitive outcomes.
Conclusions
In brief, this study lends support to the notion that depression may contribute to executive difficulties in individuals with TLE, but the types of deficits associated with depression in TLE may be distinct from those with idiopathic depression. Future studies should investigate which aspects of executive functioning improve when depression is treated in TLE.