Over two million individuals in the United States sustain a new traumatic brain injury (TBI) each year, and over five million live with TBI-related disabilities.
1 The long-term consequences of TBI include physical, cognitive, emotional, and behavioral symptoms that can persist for decades and negatively affect community participation, health, and quality of life.
2,3 Most notably, depression and behavioral dysfunction, including disinhibition, poor decision making, and apathy,
4,5 account for the majority of rehospitalizations beyond the first year postinjury,
6 result in increased medical costs, and strongly contribute to the high suicide risk after TBI.
7,8 However, the majority of individuals with TBI are not receiving adequate mental health care.
9 Timely treatment of these behavioral and emotional problems could reduce public health care burden and save lives.
Behavioral dysfunction occurs frequently (>50%) and persists after severe TBI
3; it is one of the greatest contributing factors to poor outcomes (e.g., disability, suicidality, quality of life) in this population.
10 Depression has a prevalence of approximately 50% in the first year after injury,
9 and there is a strong association between depression and behavioral dysfunction after TBI.
3 One study reported that irritability and anger, components of behavioral dysfunction,
11 were present in 54.5% of individuals in the general population with depression.
12 Neuroanatomical evidence for the mechanisms of antidepressant treatment points to shared neuroanatomy between depression and behavioral dysfunction,
13,14 particularly in the prefrontal and orbitofrontal cortexes,
15 which are associated with aggression,
16 impulsivity,
17 and executive dysfunction.
18 There is also evidence that behavioral dysfunction after TBI is more likely to occur in the context of a psychiatric disorder,
19–22 suggesting that depression may be in the developmental pathway to behavioral dysfunction.
23 Miller,
24 in discussing two recent studies that characterize depression,
12,15 goes so far as to state that “irritability, anger, anhedonia, or disruptive behavior may be equally defining [as poor mood] of the illness that we call depression” (p. 1131).
24Clearly, the nature and temporality of the relationship between depression and behavioral dysfunction remain elusive, and no study has examined their temporal relationships after TBI. We have recently developed and published a conceptual model that situates behavioral dysfunction after TBI at the intersection of an individual’s cognitive ability (e.g., executive function), emotional state (e.g., depression), and personal factors (e.g., genetics, coping skills).
22 Based on this model, we would hypothesize that changes in emotional state, such as depression, would be among the factors contributing to dysfunctional behaviors after TBI. To test this hypothesis, the purpose of this study was to examine temporal relationships between depression and behavioral dysfunction at 6- and 12-months post-TBI using a cross-lagged panel analysis structural equation model (SEM), which examines the structural relationships of repeatedly measured constructs.
25 We hypothesized that 1) depression and behavioral dysfunction would be strongly associated throughout the first year post-TBI and 2) 6-month depression would be the stronger contributor to 12-month depression and behavior.
Methods
Participants
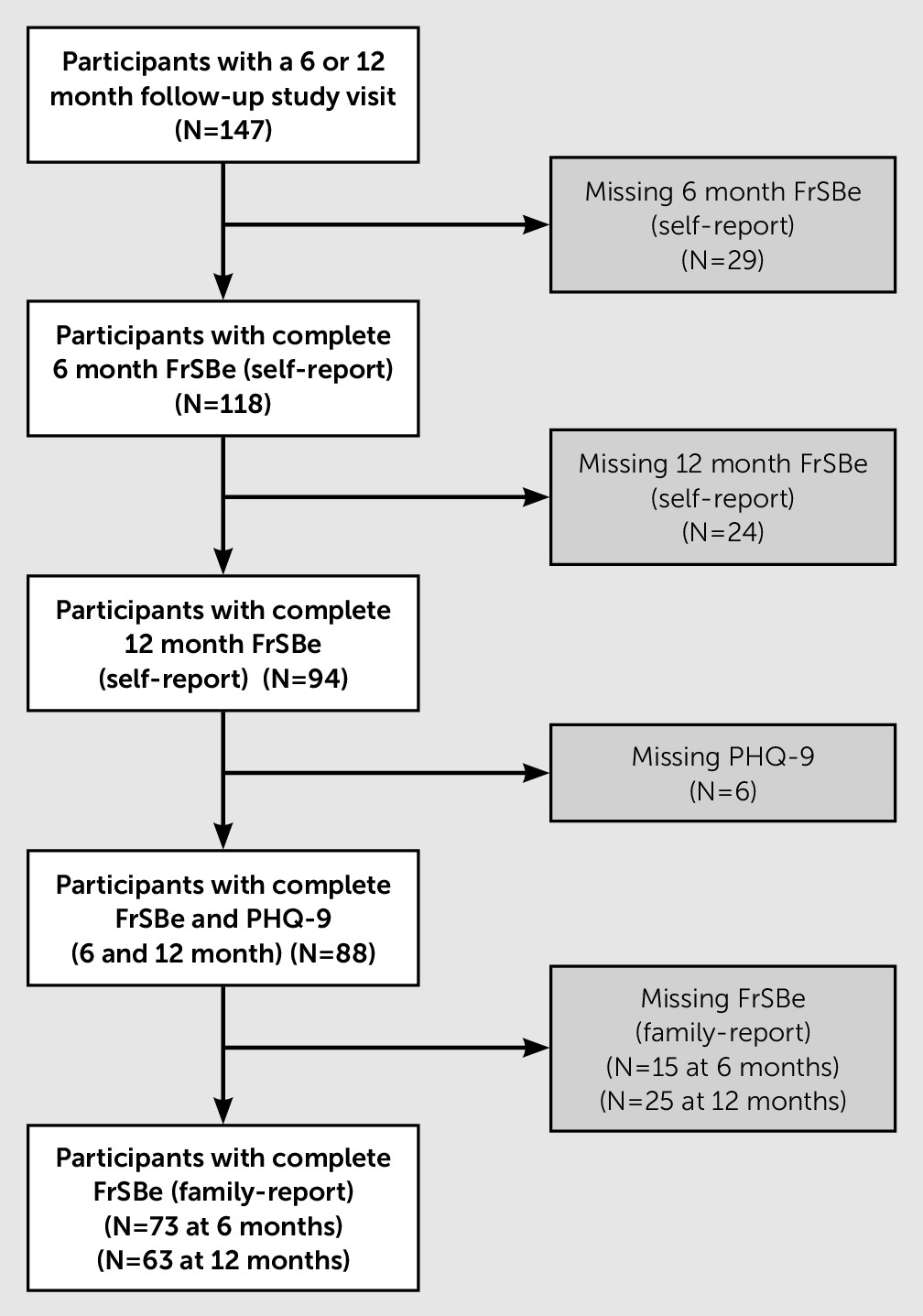
Participants in this secondary analysis (N=88) were recruited as part of two institutional review board-approved cohort studies under an umbrella protocol at the University of Pittsburgh and were recruited from both acute care and inpatient rehabilitation centers. Enrollment criteria for the parent studies were 1) nonpenetrating TBI-related
ICD-9 diagnosis or sufficient medical documentation on the day of injury (e.g., admission Glasgow Coma Scale [GCS] score ≤12, CT scan with evidence of intracranial injury, focal neurologic signs) and 2) 16–79 years old. Exclusion criteria were 1) evidence of prolonged hypoxia (>30 minutes) occurring prior to admission, 2) untreated endocrine disorder, 3) autoimmune disorder, 4) history of neurological or neurodegenerative disease, and 5) documented history of previous TBI or stroke. An additional inclusion criterion for the present analysis was to have complete behavioral and depression assessments at both 6- and 12-months postinjury (see
Figure 1). Ability to complete self-reported assessments was a prerequisite for completing the depression assessment. There were no demographic or clinical characteristic differences between included and excluded individuals with regard to sex, age, or education.
Demographic and Clinical Characteristics
Demographic (age, sex, education) and clinical characteristics (injury severity, premorbid mental health condition, antidepressant or other psychotropic medication use) were collected through a combination of participant or family member report and medical chart abstraction. Injury severity was measured through the best documented GCS score in the first 24 hours postinjury. Participants (or family members) were asked about any preinjury history of psychiatric disorder (e.g., depression, anxiety, bipolar disorder). Current medication lists were collected at both 6 and 12 months, from which antidepressants (fluoxetine, citalopram, sertraline, escitalopram, paroxetine, trazodone, duloxetine, venlafaxine, buproprion, mirtazapine, and amitriptyline) and other psychotropic medications (divalproex sodium, aripiprazole, risperidone, and ziprasidone) were extracted.
Depression
The Patient Health Questionnaire-9 (PHQ-9) is a validated self-report assessment of depressive symptoms based on
DSM-IV criteria for major depression. The PHQ-9 is validated for use after TBI
26 and can reliably discriminate between chronic TBI and depression symptoms.
27 Because of potential differences in the development of depression after TBI (e.g., adjustment to disability
28 versus inflammatory-induced depression
29), we dichotomized the scale into somatic (sleep, energy, appetite, concentration, and psychomotor slowing/agitation) and mood (little interest/pleasure, feeling down/depressed, feeling bad about self, suicidal ideation) symptoms to serve as indicators for the latent construct of depression in our SEM. For the purposes of descriptive analysis, we categorized participants as depressed or not depressed based on previously established criteria found to be optimal after TBI.
26 Briefly, participants had to endorse at least five of the nine questions on the PHQ-9 (≥1), one of which had to be question 1 (depressed mood) or question 2 (loss of interest).
Behavioral Dysfunction
The Frontal Systems Behavior Scale (FrSBe) is a validated assessment of behaviors associated with frontal lobe damage.
30 The FrSBe comprises three subscales—apathy, disinhibition, and executive dysfunction—which are assessed via self- or family-report. For the present study, self-reported assessments were used for the primary model, to be consistent with self-reported depressive symptoms. Family-reported assessments were used as a validity check to address potential limitations in self-reporting of behavior. The FrSBe produces norm-based t scores, which are adjusted for age, sex, and education. A higher t score indicates greater behavioral dysfunction. The subscales served as indicators for the latent construct of behavioral dysfunction in our SEM. The FrSBe has been found to be predictive of community integration,
31 indicating that it is a good measure of behavioral dysfunctions likely to impact disability and participation. For descriptive analysis, participants were categorized as having overall behavioral dysfunction when FrSBe total t scores were >65, per recommendation of scale developers.
32Data Analysis
All statistical analyses were performed using IBM SPSS Statistics and AMOS (version 24). Descriptive analyses were conducted for demographic and clinical data to characterize the sample. A correlation matrix was run to assess relationships between all indicator variables (depression and behavioral dysfunction at 6- and 12-months post-TBI) prior to model creation. A cross-lagged panel SEM was implemented to assess the temporal relationships between depression and behavioral dysfunction at 6- and 12-months postinjury. SEM allows for multiple relationships to be analyzed simultaneously, allowing the user to build more complex statistical models rather than running several linear regressions. The relative strengths of longitudinal relationships can be determined through comparison of standardized betas. Both depression and behavioral dysfunction were modeled as constructs (latent variables), which allowed for simultaneous inclusion of multiple observed measures (indicators) and error. Indicators of the latent variable of behavioral dysfunction include the three subscales of the FrSBe: apathy, disinhibition, and executive dysfunction. Indicators for the latent variable depression include PHQ-9 somatic symptoms and mood symptoms. Error terms were included for all indicators, and disturbance terms were included for latent variables to correct for external factors and other errors that may contribute to observed effects. Based on recommended guidelines, model fit indices were assessed, with values in parentheses indicating good fit: chi-square (p>0.05), root-mean-square error of approximation ([RMSEA] ≤0.05), nonnormed fit index ([NNFI] ≥0.90, also known as the Tucker Lewis Index), and comparative fit index ([CFI] ≥0.95).
33Results
Demographic and clinical characteristics of the 88 participants are summarized in
Table 1. On average, participants at both time points reported depressive symptoms on the low end of the mild depression range and behavioral symptoms on the high end of the normal range, though there was a very wide range within the cohort, as evidenced by the large standard deviations. These means and standard deviations are similar to those reported in a recent study in a large, multisite national database cohort represented by the TBI Model Systems.
34 Premorbid mental health conditions were reported by 18 (20.5%) participants.
At 6 months, 28 participants (31.8%) were depressed, 39 (44.3%) had behavioral dysfunction, and 21 (23.9%) had both depression and behavioral dysfunction; 34 (38.6%) were on an antidepressant medication, and four (4.5%) were on another psychotropic medication. Of the 28 participants who were depressed, 16 (57.1%) were on an antidepressant. At 12 months, 23 (26.1%) were depressed, 42 (47.7%) had behavioral dysfunction, and 18 (20.5%) had both; 30 (34.1%) were on an antidepressant medication, and three (3.4%) were on another psychotropic medication. Of the 23 participants who were depressed, 14 (60.9%) were on an antidepressant.
Table 2 reports the cross-tabulation for 6- and 12-month depression and behavioral dysfunction.
The correlation matrix (
Table 3) revealed that our indicator variables were correlated within each latent construct (r=0.560–0.690, p<0.01, for depression and r=0.468–0.769, p<0.01, for behavioral dysfunction), which ensures that the selected indicators are each representative of their respective constructs. Unlike the correlations presented later in the SEM model, these correlations do not adjust for any other factors. When evaluating across constructs, somatic symptoms of depression generally had stronger positive associations with behavioral symptoms than did mood symptoms of depression. However, the moderate-to-large correlations overall suggest that no single indicator variable is driving the relationships between latent variables (i.e., depression and behavioral dysfunction) observed in the SEM models.
All corresponding self and family subscales were significantly correlated (r=0.299–0.582, p<0.02), and discrepancies between self- and family-report, on average, were small (mean=2.88–12.10 points across subscales) and consistent with previous studies examining self-reported versus family-reported discrepancies on the FrSBe.
35SEM Model
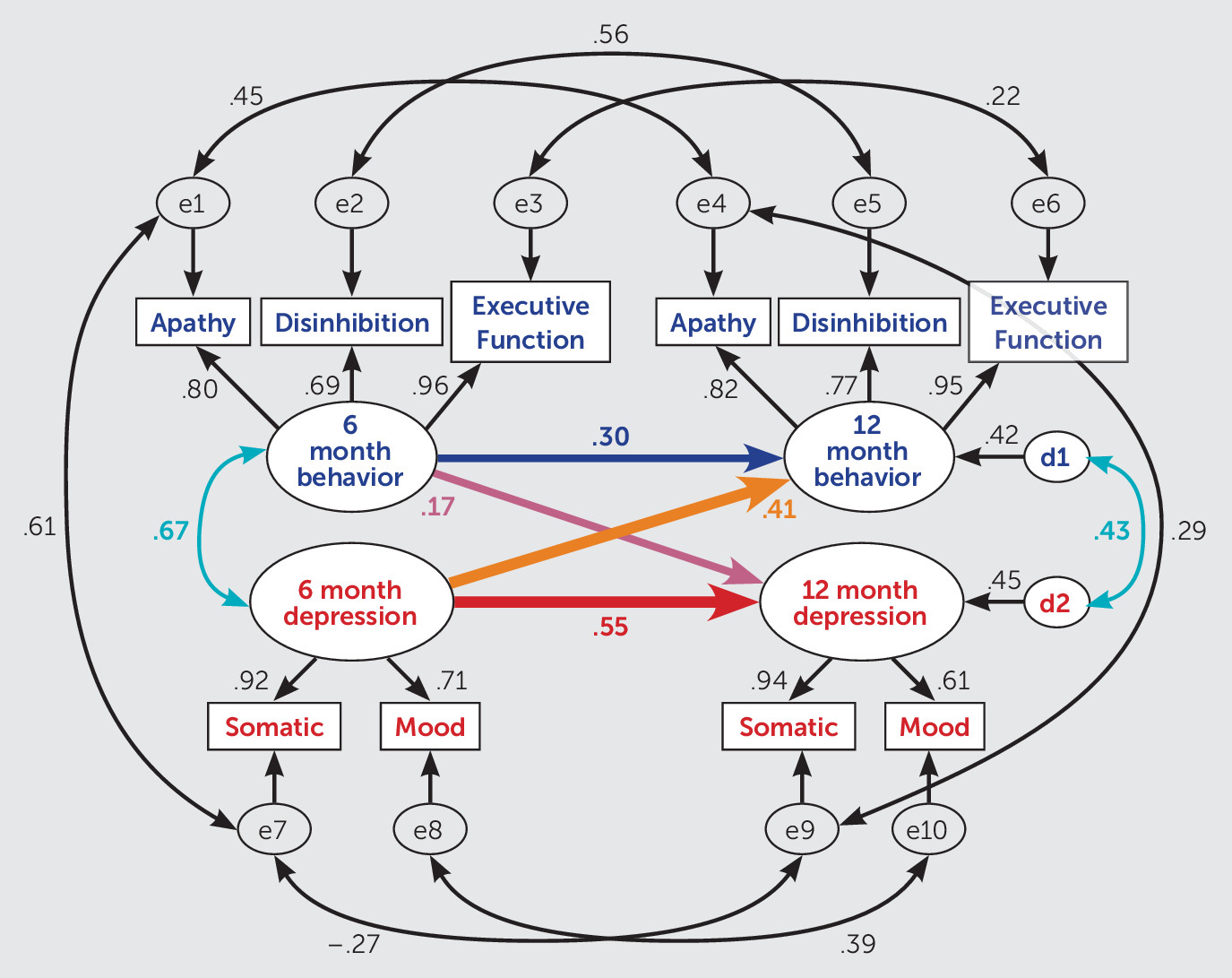
Figure 2 shows the theorized model with correlations and standardized path loadings (standardized betas). Latent variables loaded onto all of the indicators, as anticipated (all p values <0.001). Depression and behavioral dysfunction at 6 months and the disturbance (error) terms at 12 months were also significantly correlated (all p values <0.001). In addition to the a priori theorized model, the modification indices feature of SPSS Amos also prompted us to add a correlation between somatic depression and apathy to improve model fit. Given that there may be effects of fatigue, psychomotor slowing, and poor concentration (somatic symptoms) on one’s ability to initiate and engage in activities (apathy), this correlation is theoretically justified.
Latent Variable Relationships
Depression at 6 months was a significant predictor of depression (p=0.002) at 12 months postinjury and of behavioral dysfunction (p=0.004) at 12 months postinjury, controlling for 6-month behavioral dysfunction. Although 6-month behavioral dysfunction was a significant predictor of behavioral dysfunction at 12 months (p=0.035), it was not a significant predictor of 12-month depression (p=0.270), controlling for 6-month depression. The crossed path analysis showed that the strength of the relationship was stronger between 6-month depression and 12-month behavioral dysfunction (βstand=0.41) than between 6-month behavioral dysfunction and 12-month depression (βstand=0.17). This finding indicates that depression is the strongest contributor to behavioral dysfunction, but there may be some reciprocal causality over time. The total model (6-month depression and 6-month behavioral dysfunction) accounted for 42% of the variance in 12-month behavioral dysfunction and 45% of the variance in 12-month depression.
Indicators
There was a strong correlation in the SEM model between somatic symptoms of depression and behavioral apathy at 6 months (r=0.61), which likely is why inclusion of this correlation improved the model fit. Correlations between indicators at 6 months and the same indicators at 12 months were generally strong, with the exception of executive function and somatic symptoms, for which correlations were not statistically significant. These small and nonstatistically significant correlations suggest that executive function and somatic symptoms may change for some participants, but not for others, resulting in a nonlinear relationship from one time point to the next. Betas for all indicators show consistent loading of latent variables over time, which demonstrates reliability of our latent variable constructs.
SEM Fit Indices
Our overall model demonstrated good fit based on fit indices. The χ
2=26.10 (df=22, p=0.248) and the ratio of the chi-square to the degrees of freedom of 1.19 both indicate a good model fit.
36 The RMSEA was 0.046, the NNFI was 0.957, and the CFI was 0.993, also indicating good fit. When taken together, the fit statistics suggest that even this small sample size (N=88) was sufficient to produce a valid model based on a strong a priori theoretical framework and acceptable reliabilities.
33Discussion
A seminal study on the rates of depression in the first year after TBI reported that over 50% of individuals hospitalized for TBI met criteria for depression in the first year after injury, and only 44% of those individuals received either antidepressants or counseling.
9 Notably, most of the new incidence of posttraumatic depression occurred within the first 6 months.
9 However, little is known about the temporal development of behavioral dysfunction or the temporal relationships between depression and behavioral dysfunction after TBI. Timing of symptom development after injury has implications for clinical management, whether through behavioral or pharmacological intervention. Early screening and effective triage to appropriate intervention or follow-up is needed, to inform resource allocation and provide effective care for those not receiving needed services.
Our primary finding was that 6-month depression had strong effects on both 12-month depression and behavioral dysfunction, suggesting that once an individual has clinically significant depressive symptoms after injury, these symptoms can continue to self-perpetuate and lead to behavioral dysfunction. A recently published study suggests that sertraline may be effective for preventing the development of post-TBI depression,
37 which based on our findings may also prevent the development of behavioral dysfunction. When depression is present, studies indicate that dopaminergic antidepressants may be effective for addressing concurrent apathy and cognition, while serotonergic antidepressants may be better for addressing concurrent disinhibition.
38,39 However, efficacy of antidepressants for improving depression after TBI remains unclear,
40 as in the present study, in which there were participants who were depressed while currently taking antidepressants. In addition to questionable efficacy, prescribing of antidepressants after TBI is variable and often occurs for reasons other than depression. Prompt depression screening and treatment during and continuing immediately after rehabilitation discharge is critical and could have downstream effects on the development of chronic depression and behavioral dysfunction after injury.
Although depression was the strongest predictor in our model, continual evaluation of behavioral dysfunction is important as well. Only a small percentage (approximately 16%) of those with depression, behavioral dysfunction, or both at 6 months reported no significant symptoms at 12 months, and of those with both depression and behavioral dysfunction at 6 months, approximately 53% still had both at 12 months. Given the high prevalence of both posttraumatic depression and behavioral dysfunction, and the low percentage of individuals reported in previous studies to be receiving mental health treatment,
9 it is clear that there is substantial room for improvement in community-based clinical management of individuals with TBI that could substantively improve health, participation, and quality of life and decrease health care costs and economic burden of TBI.
This is the first study, to our knowledge, to examine the temporal relationships between depression and behavioral dysfunction across the first year after TBI. What is unique about TBI—or other acquired neurological conditions, such as stroke—is a defined time of onset and specific neurobiological pathology. Based on studies such as these and the extremely frequent occurrence of depression after TBI compared with the general population, it is clear that TBI is a clinical population that requires specific study to understand the causal pathways between biology, depression, and behavioral dysfunction.
This study was limited by being a secondary analysis of data from two prospective studies with common data elements and time points and by having only two data points (6- and 12-months postinjury). A prospective study design with more frequent assessment over time would provide even better information as to the best timing and targets for intervention. Although the FrSBe is an established measure of behavior associated with frontal lobe dysfunction, it does not capture all domains of behavioral dysfunction after TBI, such as anger, irritability, or aggression. A more comprehensive assessment of behavioral dysfunction would increase the generalizability of these data. A general rule of thumb for SEM analysis would suggest a minimum of 200 subjects; however, these rules can be overly conservative and not generalizable to each study in question.
41 Recent work suggests that studies with a well-developed model, high degrees of freedom, and more liberal desired power estimate (0.80) allow for SEM models to be reasonably run with sample sizes of around 140 or fewer participants.
42 Even with our a priori theoretical model and strong model-fit indices, our sample size does fall below this threshold; thus replication of these data are warranted. Though the sample size was sufficient for the analyses conducted, a larger sample size would allow for better missing data imputation and more comparisons within the SEM model, such as relationships between the individual indicators across latent constructs, and for the addition of other covariates, such as premorbid mood disorder, antidepressant use, or injury type/location. We attempted a full-information maximum likelihood data imputation method with 148 participants, and although beta values remained consistent, the model fit indices were poor, likely due to the large amount of missing data imputed.
Relying on self-report is necessary for assessing emotional and other internal states. However, there are potential limitations related to impaired self-awareness and recall bias for more objective problems such as behavioral dysfunction. Despite these potential limitations, relying on self-report is often necessary in both research and clinical settings, and we have demonstrated that self- and family-reported behavioral dysfunction were correlated in our sample. The ability to provide a self-report of these symptoms was required for this study, which meant that those with the most severe cognitive impairment in the first year after TBI who could not provide a self-report were excluded. However, those able to provide a self-report are also likely the best candidates for psychological or behavioral intervention, therefore making the study sample still representative of those whose clinical care would likely be informed by these results.
Future Directions
Ecological momentary assessment (EMA), which involves repeated measures of symptoms in real time in an individual’s natural environment, reduces reporting errors that occur as a result of poor recall or impaired self-awareness after TBI.
43 EMA of depressive and behavioral symptoms could be an effective long-term screening approach and could enable the development of temporal and potentially mechanistic pathways from injury and neurobiological changes to depression and behavioral dysfunction. Future work is needed to determine whether long-term screening improves depression treatment after TBI, whether treating depression leads to improved behavioral dysfunction over time, and whether identified risk factors for behavioral dysfunction in the context of depression could inform personalized treatment approaches.