Self-monitoring is a substantial aspect of theory of mind, as it requires evaluation and adaptation of one’s behavior to what is expected of others in order to explain and predict other peoples’ behavior. Several functional imaging studies of theory of mind have brought to light possible components of the neuronal substrates associated with self-monitoring, but the relevant information is inconclusive and contradictory. In particular, some studies suggest that the anterior paracingulate cortex is a key structure for mentalizing—the ability to read the mental states of other agents (
4,
5). However, previous neuroimaging studies have also shown activation in the anterior paracingulate cortex in additional tasks that may include various components of self-monitoring, such as visual self-recognition, (
6) autobiographical memory, (
4,
7) verbal self-monitoring, (
8) and self-generated thoughts (
9) concerning one’s own mental state rather than other people’s, but may also subserve other functions.
In addition, neuroimaging studies have shown that the superior temporal sulci are also crucial structures in the initial analysis of social cues (
10–
12). Moreover, the temporal poles have also been thought of as a store for personal and episodic memories, subserving the mentalizing process (
13–
16). Previous studies have suggested that the amygdala is also activated for rapid and automatic response to salient social stimuli (
12,
17). Baron-Cohen et al (
18). have reported increased activity in the orbitofrontal cortex, leading to the suggestion that this brain area is important for the processing of the rewards and punishments that are required for adaptive social behavior (
18). Taken together, these studies lead to the conclusion that many brain structures that are already know to subserve other cognitive functions are equally involved in self-monitoring.
Furthermore, evidence from clinical studies has shown the crucial role of specific brain areas for self-monitoring. Patients with the behavioral variant of frontotemporal dementia (bvFTD) are characterized by early, severe lack of awareness of their behavior and personality decline, and sometimes of their cognitive deficits. These deficits suggest an inability for self-monitoring, likely due to early impairment of specific brain circuits in the temporal lobe bilaterally (possibly involving basic emotion reading) and right temporal regions (ability to change one’s behavior) (
19). In particular, bvFTD has been associated with early deficits in functional connectivity of the anterior insula (AI) and anterior cingulate cortex (ACC) with the salience network (SN). As a result, socioemotional symptoms, such as loss of empathy and impaired self-awareness and emotion recognition, appear in patients with bvFTD early in the course of the disease (
20,
21). In addition, temporal damage in patients with bvFTD has been associated with the inability to identify sarcasm, whereas damage to the dorsomedial frontal cortex has been associated with failure to identify others’ intentions. Moreover, previous studies in patients with bvFTD referred to reduced perspective-taking ability due to ventromedial orbitofrontal cortex impairment (
22,
23). In contrast, patients with Alzheimer’s disease are often spared social functioning until later in its course (
24). In addition, patients with a semantic variant of primary progressive aphasia presented with loss of knowledge of social interaction scripts except for the simplest and most concrete, likely due to atrophy in the anterior temporal lobes (
25,
26). Previous studies have shown that although progressive supranuclear palsy (PSP) is mainly a motor disorder, it is commonly presented with bvFTD behavior symptoms (emotional blunting and disinhibition) and reduced socioemotional sensitivity due to the disruption of the subcortical regions with SN (
27–
29). In addition, corticobasal syndrome is also characterized by behavior and personality changes similar to those observed in frontotemporal dementia. Previous studies have shown impaired ability to recognize and express facial emotional expressions (
28,
30–
33). In view of the fact that large tracts of the temporal, frontal, and limbic brain appear to be involved in self-monitoring as well as other functions, the need arises to ascertain what structures are necessary for what aspects of self-monitoring
To test the above hypotheses, we retrospectively studied a large group of healthy controls (HCs) and patients with dementia by means of voxel-based morphometry to identify gray matter changes between the dementia and HC groups. Then, for these specific brain structures, a regression analysis model was employed to investigate the correlation between gray matter loss and lack of self-monitoring.
Discussion
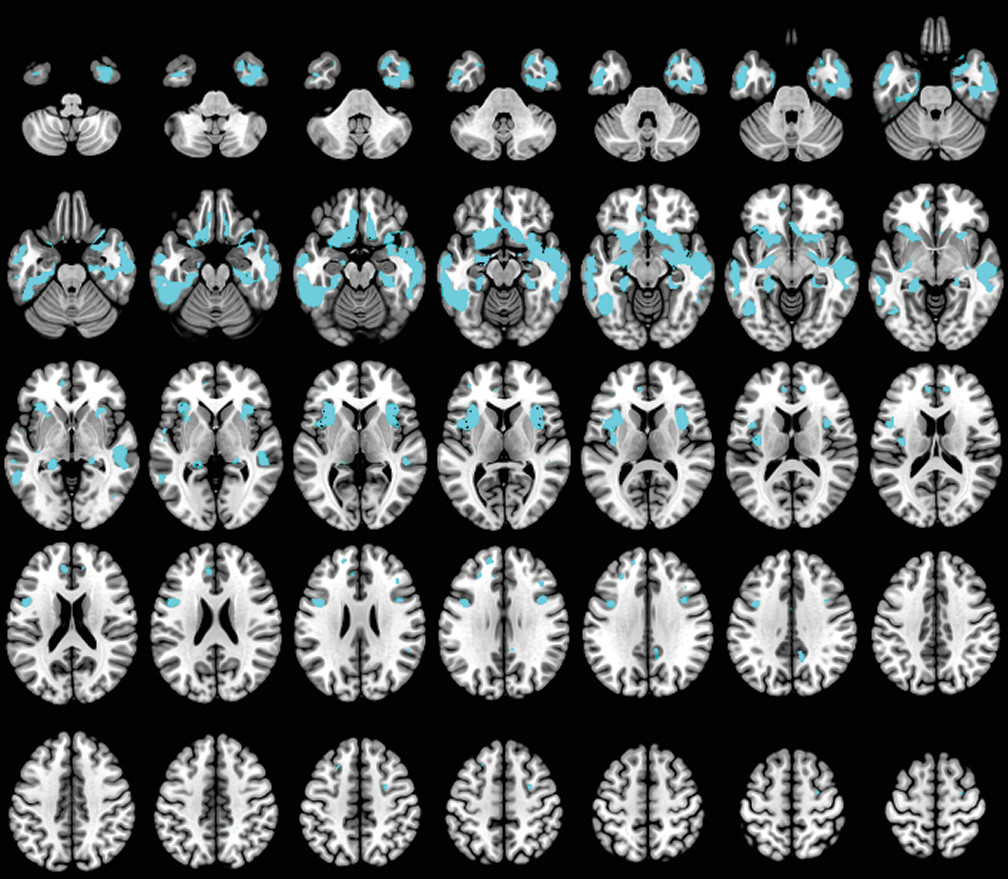
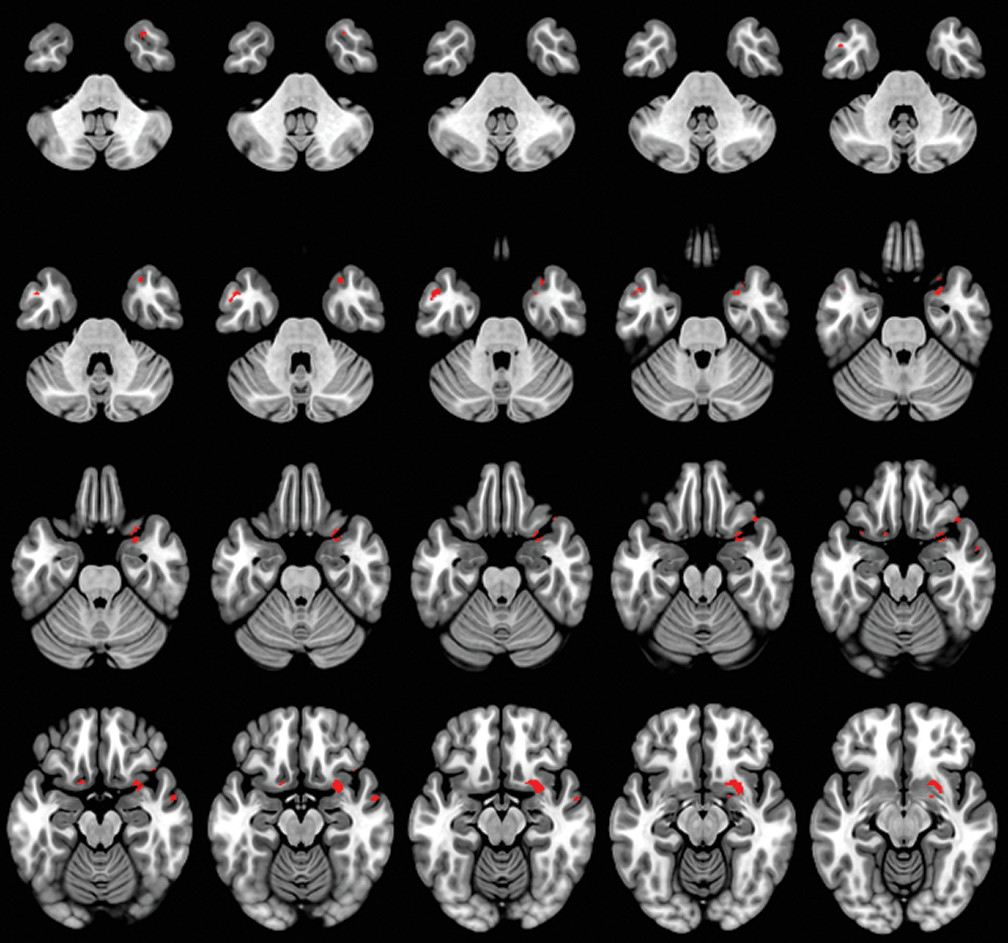
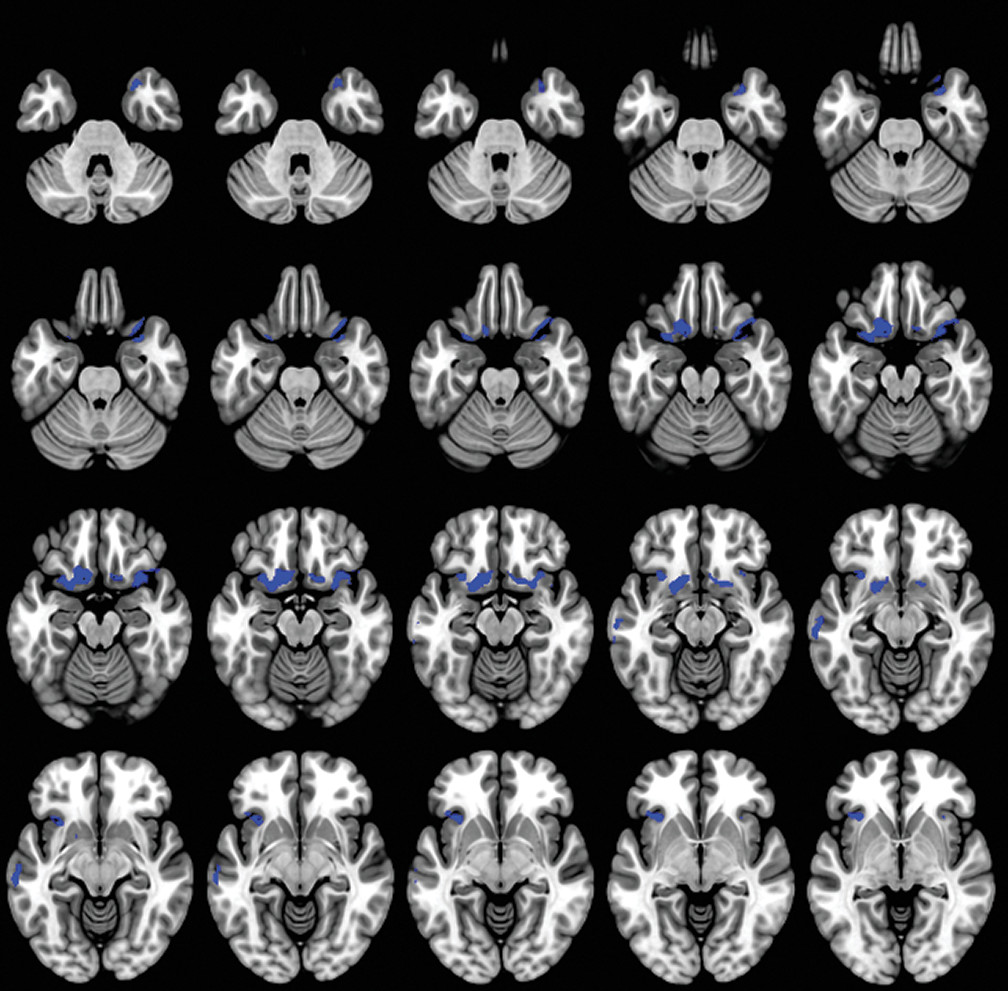
A caregiver-based measure of self-monitoring was correlated with MRI-derived gray matter density in patients with dementia. Our results confirm the gray matter changes in patients with dementia compared with the HC group (
47–
50) and also suggest that anatomical regions such as the orbitofrontal, anterior prefrontal, and temporal cortex, as well as specific limbic areas, have a crucial role in the self-monitoring function in patients with dementia. In our study, social self-monitoring loss was correlated with bilateral gray matter loss located in the olfactory cortex, rectus gyrus, inferior frontal gyrus, and insula. Additionally, it was associated with reduced gray matter density in the right temporal pole and hippocampal gyrus, and in the left superior frontal gyrus and medial temporal gyrus.
We observed that the lateral and medial orbitofrontal cortex (OFC) contributed significantly to self-monitoring in patients with dementia. The OFC, as a part of the SN, contributes to the perception of social cues and interpretation of information in the current circumstances (
21,
51,
52). In particular, (
53) the OFC is an essential brain structure in signaling the expected rewards/ or punishments for an action, given the particular details of a social frame. Furthermore, Kringelbach and Rolls (
54) have reported that medial OFC is crucial for the ongoing monitoring of the reward value of amplifiers, whereas the lateral OFC is involved in the evaluation of the punishment value of amplifiers leading to a change in the current behavior. Previous studies also indicated that orbitofrontal damage impairs self-monitoring, precluding generation of social emotions associated with the resolution of social mistakes (
55). Damage to the OFC has been associated with the ability to state the rules with a failure to apply these rules to ongoing behavior (
56,
57). Additionally, the reduced reward-related attention to social cues has been directly associated with many of the socioemotional deficits observed in bvFTD patients (
58,
59).
The insula cortex was also significantly associated with decreased self-monitoring in our study. The insula is involved in experiencing emotions and representing the emotional state of other people (
51). Additionally, the insula was considered a crucial brain structure in the evaluation of the incoming stimuli for personal and social salience (
60). In particular, the AI integrates highly processed sensory stimuli with homeostatic, affective, motivational, and hedonic information, proving a fundamental basis for emotional awareness in a social setting due to interconnectivity with SN nodes (
52,
61–
63). According to Berntson et al., (
64) damage to the insula causes reduced response to both unpleasant and pleasant visual stimuli, suggesting a role for the insula in emotional processes. In addition, it has been associated with abnormal decision-making under uncertainty and risk (
65,
66).
In our study, the anterior temporal pole was correlated with self-monitoring. Previous studies have implicated the temporal lobe in understanding social behavior, deriving social meaning, and maintaining social bonds in a continuously changing social frame (
67). In particular, the temporal pole provides supportive information about whether a stimulus is salient concerning emotional and social aspects by linking sensory representation with emotional and social memory (
68). Furthermore, it has been suggested that damage to the temporal lobe could lead to a hypoemotionality to visual stimuli and poor understanding in social words versus nonsocial words, (
6) as well as a loss of person knowledge (
68,
69). In addition, the temporal pole is a crucial brain structure for faultlessly inferring others’ intentions and thoughts by retrieving semantic and autobiographical information (
70).
The ventral medial prefrontal cortex (vmPFC) was associated with decreased self-monitoring. The vmPFC plays an important role in representation of and reasoning about others’ emotions. It has been reported that the socioemotional process between the self and the person being evaluated (self-relatedness) may be underlined by vmPFC, which probably mediates the qualities of the withdrawn information for this procedure (
71–
74). Additionally, previous research has indicated the coactivation of vmPFC, along with the default mode network, during imaging of one’s own feelings or retrieving an autobiographical memory (
71,
75–
77). In addition, activation in the subgenual ACC (sgACC) may reflect a bottom-up information sensitivity and its potential for self-other evaluation in a positive light (
78,
79). Recent studies have implicated the sgACC in detecting subjectively rewarding opportunities (
78,
80,
81) and evaluating social threats related to the self (
82). Moran and colleagues suggested that sgACC is responsive to the emotional valence of information, but only for traits that were judged to be self-descriptive (
80).
Our findings demonstrate that self-monitoring is associated with decreased brain volumes in the left dorsolateral prefrontal cortex (dlPFC) and right ventrolateral prefrontal cortex (vlPFC). According to Sollberger and colleagues, (
60) behavioral regulation in a manner appropriate to the social context is mainly mediated by dlPFC and vlPFC. In particular, composition of one’s appropriate behavior response, in accordance with the external social context, is regulated through the suppression of selfish behavior and active memory retrieval (
83) by the dlPFC and vlPFC, respectively. Decety and Jackson (
3) suggested that dlPFC mediates inferring others’ intentions and imagining others’ knowledge or feelings, thus temporarily inhibiting perspective taking. Previous studies have suggested that the damage to the dlPFC is associated with poor performance in perspective-taking tasks (
3,
84,
85). Furthermore, patients with lesions in the dlPFC also showed deficits in using social cues to make interpersonal judgments (
86).
Thus, it appears that dementia could impair both sensitivity to the expressive behavior of others and subjects’ tendency to monitor their self-presentation. Once a social cue is presented, a low-level salience detection process is performed to separate stimulus with personal relevance from noise. Lateral OFC, including the olfactory cortex and rectus gyrus, and the insula are key structures for this process (
60). It is possible that the impaired OFC in patients with dementia could lead to an inability to evaluate their behavior and compare expected reward or punishment with the delivered reward or punishment, along with a failure to perceive their social mistakes and adapt their behavior to social rules (
87). Insula damage may result in an inability to recognize and evaluate risk during decision-making, as well as identify social norm violations (
88). Furthermore, temporal lobe loss suggests the important role of this brain region in modulation of high-level social behaviors, which may be due to disruption of connectivity with other limbic regions through the uncinate fasiculus (
52,
67,
89,
90) In addition, damage to dlPFC and vlPFC may affect the ability for complex social reasoning and deliberate regulation of social behavior, causing an ineffective top-down control process to emotion-processing regions such as OFC and insula, resulting in the deficits in self-monitoring observed in our patients (
91).
In addition, the emotion sharing, emotion understanding, and emotion regulatory mechanisms required for self-other distinction during bottom-up and top-down processes of empathy involve structures mainly in the right fronto-limbic network (
92–
95). According to previous studies, lower affective and cognitive empathy were associated with smaller volume in right fronto-limbic regions, including the right hippocampus, parahippocampal gyrus, thalamus, fusiform gyrus, inferior temporal gyrus, and dorsomedial and dorsolateral prefrontal cortices in patients with fronto-temporal damage due to neurodegenerative disease (
96–
100).
Although our study has achieved its aims, there are some limitations. First, the informant reports concerning self-monitoring provided by caregivers or close relatives may be insensitive to aspects of this processing. Second, our study does not include other social and emotional measures or findings from the different dementia groups. Third, the patient group included patients diagnosed with various types of dementia; however, strict threshold p<0.05 FWE was applied to hedge the inhomogeneity of the patients’ group. Fourth, as our study conducted by means of voxel-based morphometry, specific elements of the self-monitoring instead of other social and emotional roles of the above-mentioned regions could not be identified. Future work should focus on the clarification of these aspects of self-monitoring using fMRI or single-photon emission computed tomography neuroimaging techniques.
In summary, our research has revealed that the lateral OFC, insula, temporal pole, dlPFC, and vlPFC are essential brain areas for the self-monitoring process. Damage to these areas could lead to decreased socioemotional expressiveness and modification of self-presentation aspects. Our results suggest that in patients with dementia the decreased ability for both low-level and high-level self-monitoring processes is probably due to impaired insula and OFC and their disconnection from structures in the SN (
24). These findings can not only contribute to a more accurate diagnosis but also be used to provide better care to patients with dementia.