People attribute life events to either internal or external forces on the basis of their frame of reference, which can be measured by locus of control (LOC), a dimension of an individual’s personality core self-evaluation. Individuals with a high internal LOC believe that events are a consequence of their actions and therefore within their control, whereas individuals with a high external LOC believe that other people (including doctors), circumstances, or chance dictate life events, which are therefore beyond their control (
1). Internal LOC has been associated with greater self-care and understanding of illness compared with external LOC (
2). Greater external influence of others has been identified among patients with psychogenic nonepileptic seizures (PNES) compared with patients with temporal lobe epilepsy (
3).
Functional movement disorders (FMDs) are commonly associated with disabling symptoms and poor prognosis (
4,
5). If the frame of reference of a patient with FMD is based on factors beyond his or her control as an explanation for his or her problems, the patient is thought to have a high external LOC. Conversely, if a patient believes that it is his or her fault that life problems have developed, one would expect the patient to have a high internal LOC. We sought to determine the extent to which patients with FMD differ in their internal versus external general and health-specific LOC and whether LOC affects disease severity, quality of life, and functional impairment in these patients compared with control subjects with degenerative (Parkinson’s disease) and nondegenerative (focal dystonia) neurological conditions. In this first-of-its-kind study, we hypothesized that the frame of reference among FMD patients would be dependent on factors beyond their control (high external LOC) and have a negative effect on disease severity, quality of life, and functional impairment.
Methods
Study Design and Participants
For this cross-sectional study, consecutively consenting participants were recruited between June 2015 and August 2017 from the University of Cincinnati James J. and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders and the University of Louisville Movement Disorder Clinic. All diagnoses were made and delivered by two trained movement disorders neurology specialists (A.J.E. and K.L.). Eligibility criteria were age ≥18 years, with clinically definite FMD (
6,
7), Parkinson’s disease (
8), or focal dystonia (
9). Patients who had cognitive impairment, who were pregnant, or who were unable to complete questionnaires were excluded.
This study was conducted in accordance with good clinical practice and the Declaration of Helsinki guidelines. The study protocol was approved by the institutional review boards of the University of Cincinnati and the University of Louisville. Written informed consent was obtained from all study participants.
Assessments
Frame of reference can be estimated for general aspects of life and for health-related behaviors (
10,
11); while there may be an overlap, these variables are not exchangeable (
12). Thus, all participants completed two self-administered LOC questionnaires: the Levenson Multidimensional Locus of Control Inventory (LOC-G) and the Multidimensional Health Locus of Control Assessment–Form C (LOC-H).
LOC-G is a 24-item general LOC scale. It consists of one internal (internal control) and two external (“chance” and “powerful others”) dimensions. The score range for each dimension is from 0 to 48, with higher scores indicating higher LOC per dimension (
10).
LOC-H is an 18-item health-specific LOC scale. It consists of one internal and three external (“chance,” “doctors,” and “other people”) dimensions. For the internal dimension and the chance external dimension, the score range is from 6 to 36, and for the external dimensions of doctors and other people, the score range is from 3 to 18, with higher scores indicating higher LOC per dimension (
12).
Additional measurement tools included the Toronto Alexithymia Scale-20, with a score ≥61 indicating alexithymia (
13); the Positive and Negative Affect Schedule (PANAS), a psychometric questionnaire that independently measures positive and negative affect (i.e., the response to situations in a cheerful or distressed way, respectively) and is scored from 10 to 50 for each factor, with higher scores indicating greater affect (
14); the Quality of Life Scale, a six-domain scale (material and physical well-being; relationships with other people; social, community, and civic activities; personal development and fulfillment; recreation; and independence), with a score range from 16 to 112 and with higher scores indicating greater quality of life (
15); and the Modified Work and Social Adjustment Scale, an 11-point Likert scale that measures functional impairment in work, social life, and family life domains, with a score range from 0 to 50 and with higher scores indicating greater impairment (
16). In addition, neurologists experts in movement disorders (A.J.E. and K.L.) rated FMD severity with the Psychogenic Movement Disorders Scale (PMDRS), which sums severity, duration factor, and incapacitation across body regions to produce a total score ranging from 0 to 144, with higher scores indicating greater FMD severity (
17).
Statistical Analyses
In the descriptive analysis, categorical variables were reported using frequency and proportion and quantitative variables with means and standard deviations. A log-normality transformation was applied in nonnormally distributed variables. Spearman’s rank correlation coefficients were estimated between LOC-G and LOC-H scores separately for each study group. One-way analysis of variance was used to determine differences between all groups. In the adjusted analysis, a separate multivariable logistic regression was conducted to determine LOC differences between the FMD group versus the Parkinson’s disease group and the FMD group versus the focal dystonia group. The results of the logistic regression analysis were summarized by using relative risk ratios (odds ratios), 95% confidence intervals, and p values. Exploratory post hoc unadjusted LOC mean differences between the FMD group versus the Parkinson’s disease group and the FMD group versus the focal dystonia group were conducted with Bonferroni-corrected p values. Within the FMD group, adjusted associations of LOC variables with quality of life and functional impairment outcomes were determined by using ordinary least-squares analyses. The functional impairment, a latent variable standardized score, was created with work life, social life, and family life functional impairment scores by using a structural equation-modeling approach with a maximum likelihood estimation method. Ordinary least-squares analyses were summarized with relative regression coefficients, 95% confidence intervals, and p values. Within the FMD group, we determined the impact of LOC on PMDRS scores by using ordinary least-squares analyses. Variables at the 20% level of significance in the univariate analysis were considered for multivariable analyses. A backward automated-selection approach was used for selecting important covariates in the multivariable regression models. Variables with a 10% level of significance were retained in the final model. We performed sensitivity analyses to validate the stability of the regression models. All statistical analyses were conducted with Stata 15 (StataCorp, College Station, Tex.).
Results
A total of 156 patients (FMD, N=45, Parkinson’s disease, N=64, and focal dystonia, N=47) met inclusion criteria. The mean age of the study participants was 58.3 years (SD=14.2), and the mean duration of symptoms was 4.7 years (SD=1.1). Nine patients in the FMD cohort exhibited more than one FMD. The phenotypic range of FMDs included tremor (42.2%), dystonia (42.2%), chorea (13.3%), myoclonus (11.1%), tics (4.4%), and athetosis (4.4%). Patients in the FMD group were more likely to be younger and single and to have a shorter disease duration, fewer years of education, greater alexithymia and negative affect, lower quality of life scores, and higher functional impairment in work, social, and family life categories compared with patients in the Parkinson’s disease and focal dystonia groups (
Table 1). Correlations between general and health-specific LOCs for all three study groups are summarized in Table S1 in the
online supplement.
General Frame of Reference
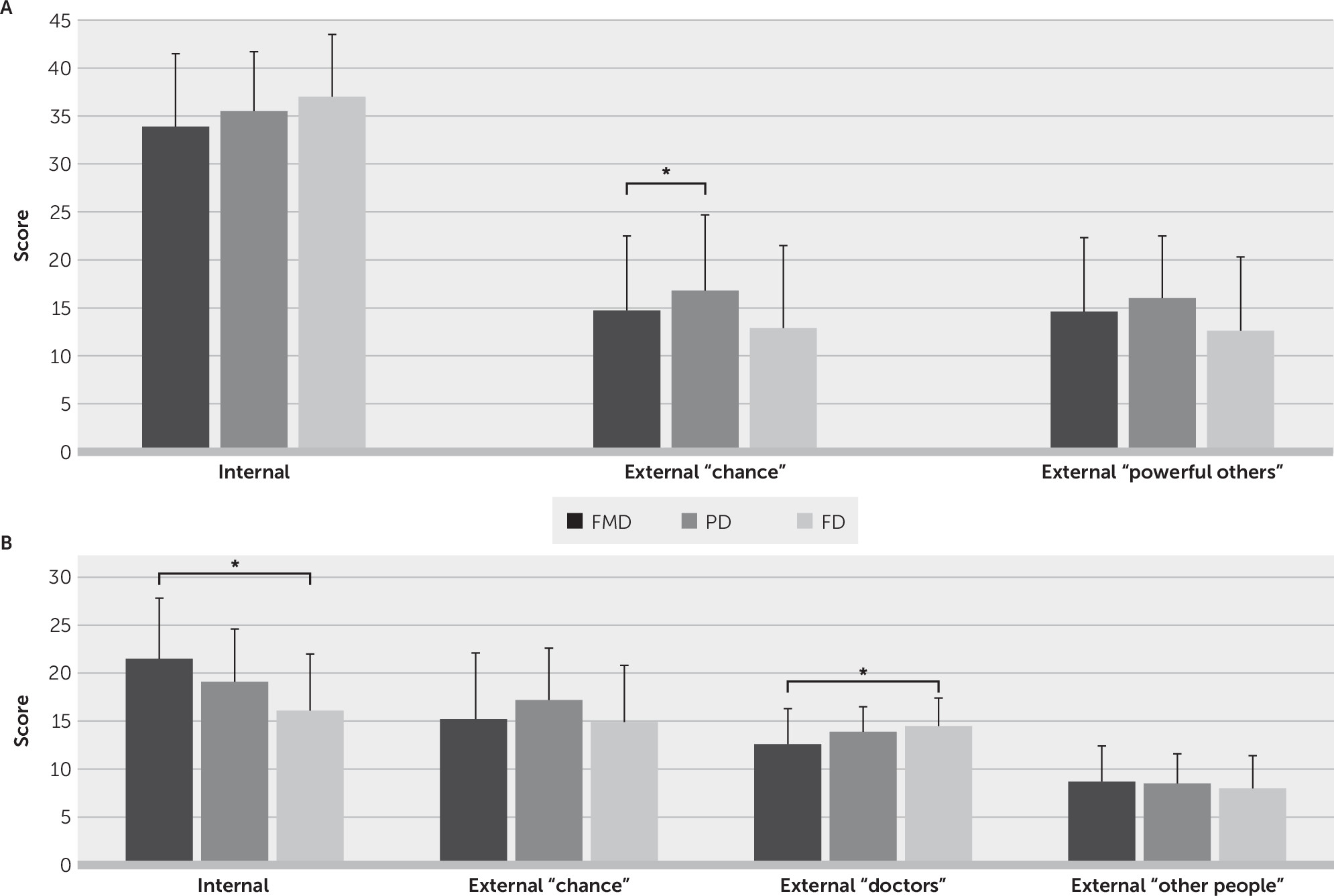
External (chance) LOC-G scores were significantly different between the FMD, Parkinson’s disease, and focal dystonia groups (
Table 1). In the adjusted logistic regression analysis, patients with FMD had lower external (chance) LOC-G scores (odds ratio=0.90, 95% CI=0.83 to 0.99, p=0.031) compared with patients in the Parkinson’s disease group only (
Figure 1A). Post hoc analysis showed no LOC-G unadjusted differences between the FMD and Parkinson’s disease groups or the FMD and focal dystonia groups (for further details, see Table S2 in the
online supplement).
Health-Specific Frame of Reference
Internal and external (doctors) LOC-H scores were significantly different between the three study groups (
Table 1). In the adjusted logistic regression analysis, patients in the FMD group had higher internal LOC-H scores (odds ratio=1.22, 95% CI=1.05 to 1.42, p=0.008) and lower external (doctors) LOC-H scores (odds ratio=0.77, 95% CI=0.62 to 0.96, p=0.02) compared with patients in the focal dystonia group only (
Figure 1B). Post hoc analysis showed significant unadjusted mean differences in internal LOC-H and external (doctors) LOC-H scores between patients with FMD and the focal dystonia group only (for further details, see Table S2 in the
online supplement).
Frame of Reference Modulation of Disease Severity, Quality of Life, and Functional Impairment in FMD Patients
The FMD disease severity score (PMDRS) was 19.5 (SD=10.7). LOC did not affect disease severity or quality of life. High external (powerful others) LOC-G scores (regression coefficient=−0.04, 95% CI=–0.08 to –0.004, p=0.029) were associated with low functional impairment.
Discussion
The novel application of examining LOC in patients with FMD revealed different internal versus external frames of reference when comparing these patients with control subjects with other neurological conditions (Parkinson’s disease and focal dystonia), with an unexpected (contrary to our hypothesis) high internal general and health-specific frame of reference among patients with FMD, which corresponds with a low external (doctor) health-specific frame of reference. These findings do not support the hypothesis of higher influence of perceived factors beyond one’s control (external) among patients with FMD, which may be implicitly assumed to be prevalent in this patient population. Also contrary to our hypothesis, the results suggest that the high internal frames of reference may not directly affect quality of life or disease severity.
As demonstrated in previous studies, greater internal LOC is associated with better understanding of one’s own disease process and self-care (
18) and even predicts spell-free intervals among patients with PNES (
19). In contrast to most patients with PNES (
3), we found that FMD patients had a significantly higher internal health-specific LOC compared with control subjects with other neurological conditions. This may be explained by distinct age, disease duration, and psychiatric comorbidity in the samples. In addition, it has been proposed that LOC metrics may be useful for individualizing treatment strategies (
11). It can be hypothesized that individuals with high internal LOC may benefit from self-directed therapies, whereas those with high external LOC may benefit from a predominantly caregiver-driven treatment. Our finding that patients in the FMD group had lower external (doctor) health-specific LOC compared with patients in the focal dystonia group may partially explain the historically poor prognosis and treatment response among FMD patients (
20), most of whom are therapist-driven to seek treatment. However, it also provides a justification for self-guided treatment options, such as Internet-delivered cognitive-behavioral therapy (CBT) (
21,
22). Nevertheless, prospective studies may identify FMD patients who may selectively respond to therapist-driven approaches (e.g., CBT).
FMD patients had the lowest quality of life scores but the highest impairment scores compared with patients in the Parkinson’s disease and focal dystonia groups. Previous studies have shown that disability and quality of life scores may be comparable between patients with FMD and those with Parkinson’s disease (
23). Our FMD cohort, however, was more likely to be unemployed, which may explain these contrary findings. In addition, high external (powerful others) general LOC was associated with low functional impairment in FMD patients. This contradicts findings pertaining to other neurological conditions, such as Parkinson’s disease and multiple sclerosis, in which high scores for internal rather than external LOC have been associated with reduced functional impairment (
1,
2).
Our study has some limitations that affect the strength of our conclusions. First, we did not screen for anxiety or depression, which are highly prevalent comorbid conditions among patients with FMD (
23,
24). It is plausible that these psychiatric comorbidities may have an effect on quality of life, functional impairment, and severity metrics. Second, we did not include a healthy control group nor a functional control group in which the LOC might have a different influence. Third, we did not assess additional self-evaluations of core personality traits, such as generalized self-efficacy and self-esteem (
25). These self-evaluations may modulate quality of life, functional impairment, and severity in FMD patients. Finally, the cross-sectional design precludes drawing any conclusions about the effect of personality estimates in prognosis or treatment response or any potential changes to LOC, even if it is considered to be generally stable across time.