Cannabis is said to be a component cause of schizophrenia, elevating the risk of psychosis to two to three times that of the general population (
1–
3). In a substantial proportion (55%–60%) of patients, psychosis is time limited and resolves completely in one to six months (
4,
5). Current classification systems label it as cannabis-induced psychosis (CIP) (
6,
7). The other group (40%–45%) of patients experience psychosis that transitions to schizophrenia spectrum disorders in three to seven years. There is a significant overlap between the initial presentation of CIP and the group with schizophrenia with cannabis use (SZC) (
8,
9). However, a careful assessment at presentation with specialized interview tools, such as the Psychiatric Research Interview for Substance and Mental Disorders (PRISM), can differentiate the CIP and SZC diagnoses.
The question is whether there is any difference between CIP and SZC at the neurobiological level. A recent study showed that people with SZC have more impaired general cognitive ability and attention compared with people with CIP. Moreover, SZC has demonstrated a higher degree of cognitive impairment than CIP when compared to a control group (CG) (
10). These results suggest that neuroimaging may help to further differentiate these two clinically overlapping forms of psychosis. Prior literature has not examined the neurobiological characteristics of CIP and compared them with those of SZC. Nevertheless, neuroimaging studies have examined the white matter (WM) microstructure and gray matter (GM) volume of patients with schizophrenia and co-occurring cannabis use and compared them with those of patients with schizophrenia who do not use cannabis (or any other substance). A study by Quinn and colleagues (
11) reported no significant differences in GM volumes in various frontal, temporal, occipital, and cerebellar regions between patients with schizophrenia with or without cannabis or alcohol use. Nevertheless, the volumes were less in the schizophrenia group (irrespective of cannabis use) than in the healthy CG. Similarly, Cohen et al. (
12) showed that the cerebellar GM volume deficit did not differ between schizophrenia groups (i.e., with or without cannabis use). These findings indicate that cannabis use may not contribute to GM volume reduction in patients with schizophrenia. However, another study comparing GM volume and WM fractional anisotropy (FA) showed greater reductions in both parameters among patients with SZC than among patients with schizophrenia without cannabis use. Among WM tracts, the brain stem, internal capsule, corona radiata, and superior and inferior longitudinal fasciculi were found to be involved (
13). Therefore, the results of that study seem to contradict the observations of the two previously cited studies. Overall, published literature appears equivocal about cannabis-induced brain structural abnormalities in individuals with schizophrenia. These studies cannot help us understand why, despite a common history of chronic, heavy cannabis use, one group of individuals develops short-lasting psychosis (CIP) and the other goes on to develop schizophrenia (SZC).
In this study, we examined whether differences in GM volumes and WM structural connectivity of various fiber tracts between CIP and SZC could explain the differences between the two psychiatric disorders. Additionally, to explore the degree of structural abnormalities in both groups in reference to the general population, we included a CG without any substance use or psychosis. We included all relevant WM fiber tracts (i.e., the association, limbic, projection, and commissural fibers) and assessed microstructural integrity by measuring FA (
14). We chose GM structures on the basis of a published review on volumetric analysis of brain structures among patients with schizophrenia (
15). We hypothesized that on direct comparison, the extent of abnormalities in the FA of various WM tracts and GM volumes would be greater in SZC than in CIP, and that compared to the CG, the extent of brain changes would be greater in SZC than in CIP.
Methods
Design
The study had a cross-sectional and observational design and was conducted in a tertiary hospital–associated addiction treatment center. Three groups with 20 participants each were recruited via convenience sampling: a healthy CG, a CIP group, and an SZC group. All participants were right-handed men between 18 and 45 years of age and were matched at intake for age, education, and handedness. Study recruitment began in July 2017 and continued through June 2019, a period of 24 months.
The study was approved by the Postgraduate Institute of Medical Education and Research ethics committee for human research. Written informed consent was obtained from all participants.
Participants
The participants were included on the basis of selection criteria that involved assessment with the following instruments: a sociodemographic and clinical profile sheet, the Edinburgh Handedness Inventory, the Mini-International Neuropsychiatric Interview (MINI), the PRISM, and the Positive and Negative Syndrome Scale (PANSS) (
16–
20). For further details on clinical assessment, see the text in the
online supplement.
The inclusion criteria for the CIP and the SZC groups included early initiation of cannabis use (before age 17); long-term heavy cannabis use (more than three years and at least two times per month); diagnosis of pathological cannabis use or dependence and no diagnosis of any other axis I psychiatric disorder, as per the MINI; and a negative urine screen for opioids, benzodiazepines, barbiturates, amphetamines, and cocaine prior to assessment. Those in the CIP and SZC groups also had to have a diagnosis of cannabis-induced psychotic disorder or schizophrenia, respectively, as per the PRISM (
16). According to the PRISM, episodes of cannabis-induced psychotic disorder should occur entirely during a period of heavy cannabis use or within the first four weeks after cessation of use, and the symptoms should be greater than the expected effects of intoxication or withdrawal. Symptoms with CIP are expected to resolve significantly within the first month of abstinence and completely within six months of abstinence. Primary schizophrenia can be diagnosed in the presence of cannabis use in three specific scenarios: patients who developed schizophrenic psychosis and started cannabis use afterward; patients whose first episode of schizophrenia-like psychosis occurred during heavy cannabis use but continued beyond four weeks after cessation of cannabis; or patients whose first episode occurred during intoxication but resolved with cessation, but who then developed symptoms later in life, independent of cannabis use. Thus, participants were included in the CIP group only if a primary disorder was ruled out and at least six months had passed since the onset of psychosis. Similarly, additional inclusion criteria for the SZC group were illness for less than five years and remission in psychosis, on the basis of the PANSS criteria recommended by the Remission in Schizophrenia Working Group (
20). We have included only first-episode schizophrenia.
Exclusion criteria for both groups were presence of lifetime use of or dependence on alcohol or any category of illicit drugs other than cannabis (except tobacco), lifetime presence of other axis I disorders, organic conditions etiologically relatable to the psychosis or contributing to structural brain changes (head injury with loss of consciousness, epilepsy, and serious systemic illnesses), neurological disorders that may affect brain structure, and intelligence score less than 70.
The healthy CG included participants who were biologically unrelated to the patients, had no lifetime use of cannabis or any other illicit drug (except tobacco), had no diagnosis of an axis I psychiatric disorder (as per the MINI), had no organic conditions or neurological disorders that could affect brain structure, and had a negative urine screen for opioids, benzodiazepines, barbiturates, amphetamines, and cocaine prior to assessment.
Measures
The study measures involved structural brain imaging, including volumetric magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), and diffusion kurtosis imaging (DKI).
Image acquisition.
All MRI data were acquired on a Siemens full-body, 3-T Verio system. For further details on image acquisition, please see the text in the
online supplement.
Diffusion imaging.
Full-brain DKI sequences were acquired with a diffusion-sensitized dual spin-echo prepared echo-planar imaging sequence. Further details about DTI parameters are provided in the
online supplement.
Volumetric imaging.
T1- and T2-weighted images were used for volumetric analysis, including voxel-based morphometry of the whole brain and specific regions of interest (ROIs) as described below.
MRI data processing.
Data preprocessing followed a standard DKI processing pipeline and included visual inspection of images for quality assessment and removal of artifacts, image smoothing with a three-dimensional Gaussian filter, correction for motion and eddy current distortion, correction for distortions from B0 field inhomogeneities, and adjustment of the encoding gradients’ matrix for rotations during the motion correction step (
21,
22). For further details, please see the
online supplement.
MRI Data Metrics
Diffusion imaging metrics.
The standard diffusion metrics, derived through postprocessing with the diffusional kurtosis estimator, are axial diffusivity, radial diffusivity, mean diffusivity (MD), FA, axial kurtosis, radial kurtosis, and mean kurtosis (MK). As per the study protocol, MD and FA for diffusion tensors and MK and FA for kurtosis were included in analyses. The ROIs analyzed are defined in
Table 1.
Volumetric imaging metrics.
Brain structures for volumetric analysis, including GM ROIs and lateral ventricles, were selected for comparison between study groups. For further details, refer to the text in the
online supplement.
Analyses
Sociodemographic and clinical data were analyzed using a chi-square test (or Fisher’s exact test) for categorical variables and an independent-samples t test or analysis of variance (ANOVA) for continuous variables. For comparing FA, MD, and MK values across the WM fiber tracts, an ANOVA with a post hoc Scheffé test was done. The latter should have minimized the possibility of type I error due to multiple comparisons (
23). General linear modeling was used for comparing GM volumes among the three groups. The whole-brain volume was used as a covariate, and the Bonferroni test was used for multiple comparisons. All calculations were done in SPSS, version 16.0 (
24).
Results
A total of 79 right-handed men were asked to participate in the study; among these, 60 participants (20 in each group) were included in the final analysis. Reasons for exclusion (N=19) included not giving consent, not attending follow-up assessments, a positive urine screen for cannabis and other drugs at assessment, an intelligence score less than 70 on the Standard Progressive Matrices, and high PANSS ratings at assessment. Mean±SD time since diagnosis was 15.87±11.5 months for CIP and 42.26±15.3 months for SZC.
Comparison of the Sociodemographic Profile
There were no significant differences in age, level of education, religious background, and family type among the groups. The participants’ sociodemographic profiles are reported in Table S1 in the
online supplement.
Clinical Profile Related to Cannabis Use
There was no significant difference between the CIP and SZC groups with regard to the frequency of cannabis use during the six months prior to onset of psychosis and with regard to the preparation of cannabis used (charas, ganja, or bhang).
Table 2 presents the clinical profiles of these groups.
Comparison of Diffusion Parameters (DTI and DKI)
The comparison of diffusion metrics among the study groups revealed significant differences in MD in the WM fibers on both sides of the posterior corona radiata. The MD for the CIP group was higher than that for the CG on both left (p=0.045) and right (p=0.048) sides. The comparison also revealed a significant intergroup difference in FA on the left side of the corticospinal tract (CST). FA for the CIP group was significantly higher than FA for the SZC group (p=0.046). Significant intergroup differences were also observed in FA on both sides of the anterior limb of the internal capsule (ALIC). The post hoc test showed that FA in the ALIC in the SZC group was significantly less than in the CG (left side p=0.036, right side p=0.003). The comparison showed that FA on the right side of the retrolenticular limb of the internal capsule (RLIC) was lower in the SZC group than in the CG (p=0.025). In the SZC group, MD in the RLIC was higher than in the CG (left side p=0.002, right side p=0.018) on both sides, whereas in the CIP group it was higher than in the CG only on the right side (p=0.035). The comparison of diffusivity in the cingulate gyrus hippocampal bundle showed more FA and MD on the right side in the SZC group than in the CG. A significant increase in MD was seen in both the CIP and SZC groups in the right superior longitudinal fasciculus (SLF) fibers (CIP p=0.004, SZC p=0.042). Increased MD was seen in the left SLF fibers in the CIP group compared with the CG (p=0.012).
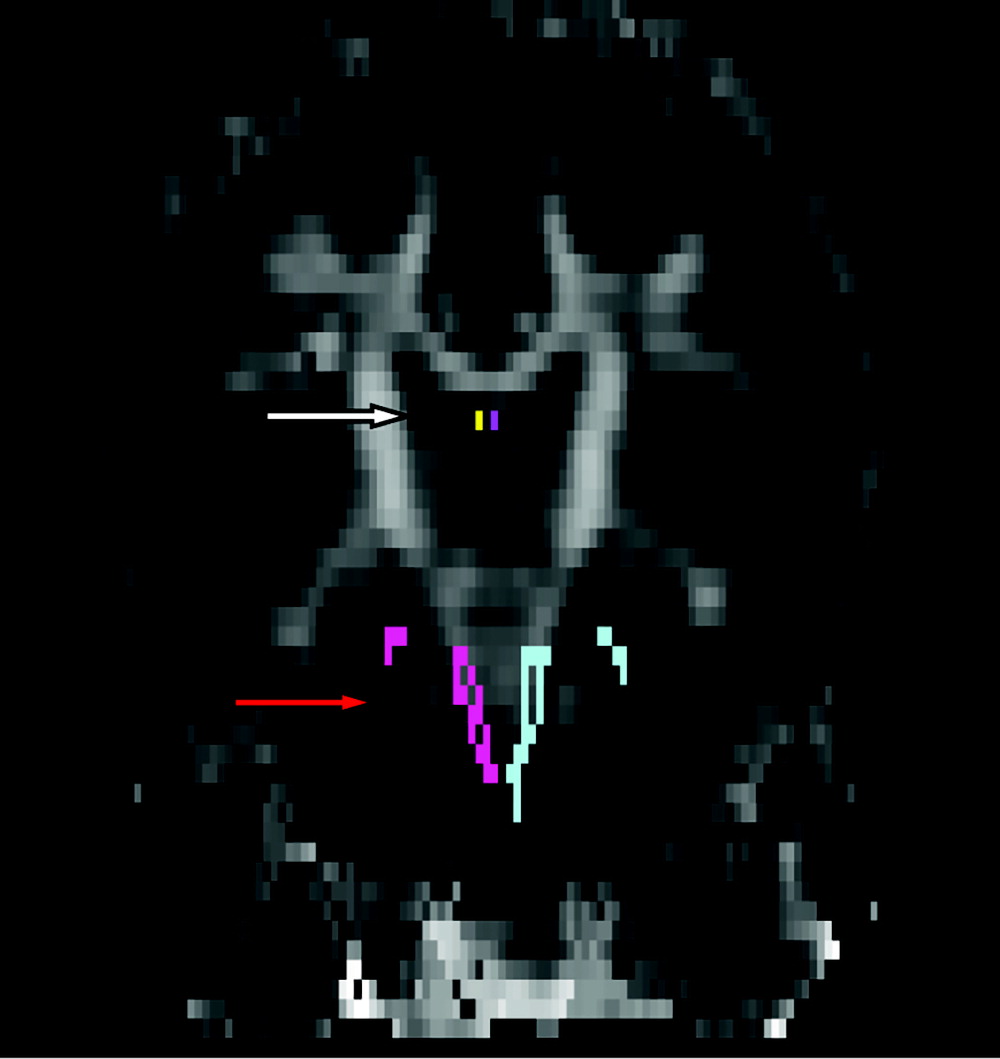
No significant group differences were observed in the DTI parameters (FA, MD) in any other WM tract (
Figure 1). Please see
Table 3 for details.
Comparison of Brain Morphometric Parameters
The comparison of brain volumes with ANOVAs among the study groups revealed significant differences in mean cerebellar volume in the CIP group (143,191.7 mm3), CG (139,932.6 mm3), and SZC group (130,477.6 mm3) (p=0.026). Post hoc testing revealed that the mean cerebellar volume of the CIP group was significantly greater than that of the SZC group (p=0.038). The finding of higher mean cerebellar volume in the CIP group than in the SZC group remained significant (p=0.047) after controlling for total brain volume. Pairwise comparisons of mean cerebellar volumes showed a trend toward the greatest volume being in the CIP group, followed by the CG and the SZC group.
In addition, there was a significant difference among groups in the volume of the right inferior frontal gyrus (IFG) pars triangularis (p=0.015). The difference remained significant after controlling for whole-brain volume; the mean volume of the CIP group was significantly higher than that of the CG (p=0.018). There was a significant difference in the mean volumes in the IFG pars orbitalis, but the difference did not remain significant in the pairwise comparison after correction for the effect of whole-brain volume with general linear modeling (p=0.054).
The differences among groups were not significant in the other GM areas. Please refer to
Table 4 and Tables S2a, S2b, and S2c in the
online supplement.
Discussion
To the best of our knowledge, this is the first study on brain structural connectivity and GM volume among people with CIP. We also had an SZC group and a CG without any substance use or psychosis. Although we used several measures to examine WM microstructural integrity, we based our interpretations on FA and used other parameters (e.g., MD, MK) as supportive indices. We observed that participants in the SZC group had worse WM microstructural integrity (lower FA) than controls in the ALIC and RLIC, cingulate gyrus hippocampal formation, fornix, and superior fronto-occipital fasciculus. WM structural abnormality in the CIP group was limited to the left fornix and right superior fronto-occipital fasciculus. On direct comparison, the CIP group had better structural integrity in the left CST and larger cerebellar GM volume than the SZC group. The CIP group had greater IFG volume than the CG. Group-level matching was done for potential effect modifiers such as age, handedness, and years of education (
25,
26). Cannabis use–related confounding factors such as age at initiation and duration and frequency of cannabis use did not differ between the CIP and SZC groups (
27). The duration of psychosis for all participants in the SZC group was less than five years and unlikely to exert a significant neurotoxic effect (
28). Further, participants with possible confounders such as other substance use, HIV infection, head injury, and seizures were excluded from the study. Therefore, the differences detected between groups of participants were unlikely to be influenced by known confounders and effect modifiers.
The group with SZC was the most negatively affected among the three groups in terms of the extent and severity of WM structural disconnectivity. Structural disconnectivity, as assessed by significant reduction of FA, was spread across the association (bilateral superior fronto-occipital), projection (ALIC and RLIC), limbic (fornix and cingulate), and brain stem fibers (CST). The increase in MD in most of these fiber tracts supported the findings of reduced FA and impaired WM integrity (
29). The WM structural network observed in the SZC group is comparable to what has been reported in the available literature. A systematic review of 17 studies examining the early stage of schizophrenia (without cannabis use) and one study of SZC showed widespread reductions in FA values across the association, projection, limbic, and callosal fibers (
14). The results of our study were similar, except that we did not find any abnormality in callosal fibers. However, this finding was not unusual, because callosal sparing has also been reported by several other studies (
30–
32). The extent of involvement in terms of the number of WM fiber tracts with impaired microstructural integrity was less in the CIP group than in the SZC group. Additionally, participants with CIP had better WM integrity in the left CST compared to those with SZC. On one hand, our results support the disconnectivity hypothesis of schizophrenia (i.e., the neural pathology of schizophrenia involves abnormal or suboptimal communication between functional brain regions and disruptions in the underlying WM structural organization) (
33–
35). On the other hand, less disrupted WM fibers in the CIP group may explain why, despite a similar degree of exposure to cannabis, the CIP group developed only short-lasting psychotic symptoms that did not evolve into schizophrenia. Moreover, better WM integrity of the CST in the CIP group may also have played a protective role and prevented the transition to schizophrenia. This hypothesis is supported by previous tract- and voxel-based analyses of WM integrity in schizophrenia spectrum disorders that have found lower FA in the bilateral CST in schizophrenia (
36,
37). Finally, although the disruptions in WM tracts were limited in the CIP group, one must not ignore the abnormalities in two of the fiber tracts; these abnormalities may increase the propensity of those in the CIP group to develop psychosis.
Analysis of GM volumes did not show any significant differences between the SZC group and CG. Although this result is counterintuitive, we can suggest some reasons for it. First, all patients in the SZC group had a short duration of illness, and existing evidence suggests progressive loss of GM volume in schizophrenia (
28). Second, a meta-regression analysis with substance use as a predictor variable reported a trend toward a significantly smaller effect size of difference in brain volume compared to schizophrenia without history of substance use (
15). The sample size of our study may not have been adequate to detect significant differences of smaller effect sizes. The greater cerebellar volume in CIP than in SZC reveals an important insight. The cerebellum is a CB
1 cannabinoid receptor–rich region, and CB
1 receptors play a significant role in synaptogenesis, synaptic pruning, morphogenesis, and neuronal migration during adolescent brain development (
38,
39). Previous research has consistently reported lower cerebellar volume in first-episode schizophrenia than in the general population and patients with bipolar disorder (
12,
40). Lower cerebellar volume in first-episode schizophrenia suggests a neurodevelopmental abnormality and may affect the propensity to develop schizophrenia. Therefore, a larger cerebellar volume in those with CIP might have provided a neurodevelopmental protection against schizophrenia and prevented the transition from short-lasting psychosis to schizophrenia, in spite of the toxic exposure to cannabis. The higher GM volume of the IFG in the CIP group is also a noteworthy finding. Previous reports have consistently shown decreased GM volume of the IFG and a relative sparing of the superior and middle frontal gyrus among patients with schizophrenia, both in a treatment-naïve first-episode group of patients and among those with chronic schizophrenia (
41–
44). Decreased IFG volume was also found in the unaffected siblings of patients with schizophrenia (
45). Taken together, these results may suggest that IFG abnormality predates the onset of schizophrenia or may be considered to confer vulnerability. Therefore, the higher IFG volume in the CIP group may have protected this group of individuals from schizophrenia.
We used DKI to supplement DTI. DKI is said to be more useful in assessing WM tracts with complex fiber arrangements (e.g., the corona radiata) (
46). In fact, the only significant difference was seen between the CIP group and CG in the MK of the left anterior corona radiata, suggesting impaired WM integrity in CIP. However, the extent and severity of WM disruptions detected with DKI were considerably less than those observed with DTI. Nevertheless, this finding is in line with the only study that compared DTI and DKI parameters in patients with schizophrenia, and that study reported that DTI detected significantly more WM abnormalities than did DKI (
46). Our study also suggests that DKI adds very little to the DTI analysis and may only be useful for WM fibers with complex arrangements.
We did not design our study to determine the potential cause of brain morphometric abnormalities and structural disconnectivity. The widespread changes in FA in SZC and limited changes in CIP might also have resulted from chronic exposure to cannabis. Previous research showed that heavy use of cannabis may be associated with a reduction in FA in the frontal tract, thalamic tract, and CST (
47). However, other studies did not find any association between brain morphometric and connectivity changes and cannabis use in developing brains (
48,
49). Hence, the existing evidence supports a higher possibility of psychosis-induced brain changes as a result of psychosis vulnerability than of brain abnormalities resulting from chronic cannabis use.
Our study has several limitations, including its cross-sectional study design. To address this concern, we used PRISM and the criterion of remission after six months to differentiate between CIP and SZC. However, a longitudinal study design would have provided a more reliable diagnosis of CIP, because CIP may evolve into schizophrenia even after six months. Another concern was the convenience sampling, which is prone to sampling bias. Therefore, our sample might not be representative of the entire population. This could result in limited generalizability of our results. The sample size in each group was not calculated a priori. However, as per Cookey and colleagues (
14), a sample size of 20 minimizes the influence of noise discoveries. Nevertheless, we understand the need to replicate our study with a larger sample. We used post hoc Scheffé and Bonferroni tests to minimize the chances of false-positive associations with the DTI parameters and morphometric parameters, respectively. In spite of these precautions, issues associated with multiple comparisons could not be ruled out. The absence of a group with schizophrenia without cannabis use and one with only cannabis use (without psychosis) prevented us from examining the full continuum of psychosis and cannabis use. However, our study had a limited objective: to examine the differences in brain structural connectivity and morphometry in CIP and SZC. Nevertheless, we cannot say whether these differences might have been caused by psychosis or cannabis use or both. Future studies could examine these questions with a larger sample, a longitudinal design, and the use of all four groups of participants.