Catatonia is a psychomotor syndrome characterized by a state of reduced responsiveness and inability to move normally despite complete physical capacity (
1). It is recognized to occur in a variety of mental disorders and reportedly affects 5%−18% of patients with acute severe psychiatric illness (
2). It may also occur in the context of many neurological and general medical disorders (
3). It is a serious and debilitating condition, associated with multiple life-threatening complications, yet it is often underdiagnosed (
4).
Catatonia was previously classified as a subtype of schizophrenia and organic mental disorders; however, the ICD-11 now recognizes catatonia as a distinct neuropsychiatric syndrome, and DSM-5 permits a diagnosis of catatonia in the context of any psychiatric or general medical disorder (
5,
6). Despite catatonia’s diverse etiologies, the generally positive response to benzodiazepines regardless of the underlying cause provides some evidence for a unified disorder (
7). Nonetheless, the pathophysiology of catatonia has remained elusive: theories about the neurotransmitters GABA, dopamine, and glutamate, as well as hypotheses regarding phenomenology, neural networks, and neuroinflammation, remain unconfirmed (
8–
13).
Findings from neuroimaging studies in catatonia have been inconsistent. Focal lesions associated with catatonia have been identified in sites as diverse as the frontal lobes, parietal lobes, temporal lobes, basal ganglia, anterior cingulate gyrus, thalamus, pons, and cerebellum (
14). A recent systematic review reported that most patients with catatonia who had abnormalities had diffuse and focal white matter lesions, occurring in many different regions (
15). Furthermore, functional imaging frequently showed frontal, temporal, or basal ganglia hypoperfusion, and structural imaging mostly showed diffuse cerebral atrophy (
15). However, most brain imaging studies in catatonia are case reports or series describing findings in a small sample of patients. Although these studies are of interest, the samples are not large enough to identify patterns, they are prone to selection bias, and the studies have often lacked comparison groups. To date, no studies have explored the structural neuroimaging findings in a large population of patients with catatonia with an appropriate comparison group.
Here we present a study that utilized a large data set to describe neuroimaging findings in clinical magnetic resonance imaging (MRI) reports, comparing them with findings for psychiatric patients without catatonia. We had two specific objectives: to identify the distribution of abnormalities among patients with catatonia in terms of laterality, localization, and pathology and to compare the frequency of such MRI abnormalities in psychiatric patients with and without catatonia.
Methods
Study Design
This study was a case-control study comparing neuroradiological abnormalities in clinical reports of MRI brain scans of patients with catatonia with those of psychiatric patients without catatonia. Anonymized electronic health care records from patients seen in the South London and Maudsley National Health Service Foundation Trust (SLaM), London, were accessed through the Clinical Records Interactive Search (CRIS). The CRIS system has previously been described (
16) and is approved by the Oxfordshire C Research Ethics Committee (ref. 18/SC/0372). This specific study was approved by the CRIS Oversight Committee (ref. 17–102).
Data Availability
Data are owned by a third party, Maudsley Biomedical Research Centre Clinical Records Interactive Search (CRIS) tool, which provides access to anonymized data derived from SLaM electronic medical records. These data can be accessed only by permitted individuals from within a secure firewall (i.e., the data cannot be sent elsewhere). (For more information, contact
[email protected].)
Outcome
Cases of catatonia were defined as having a clinician diagnosis of catatonia and at least two features on the Bush-Francis Catatonia Screening Instrument, a reliable and validated instrument for the detection of catatonia (
7,
17,
18), as described in previous work by this group (
19). Because this was a heterogeneous population with a range of diagnoses, the comparison group comprised all patients admitted to psychiatric wards in the Trust who had never had a catatonia diagnosis. All patients with catatonia had also been psychiatric inpatients.
Exposure
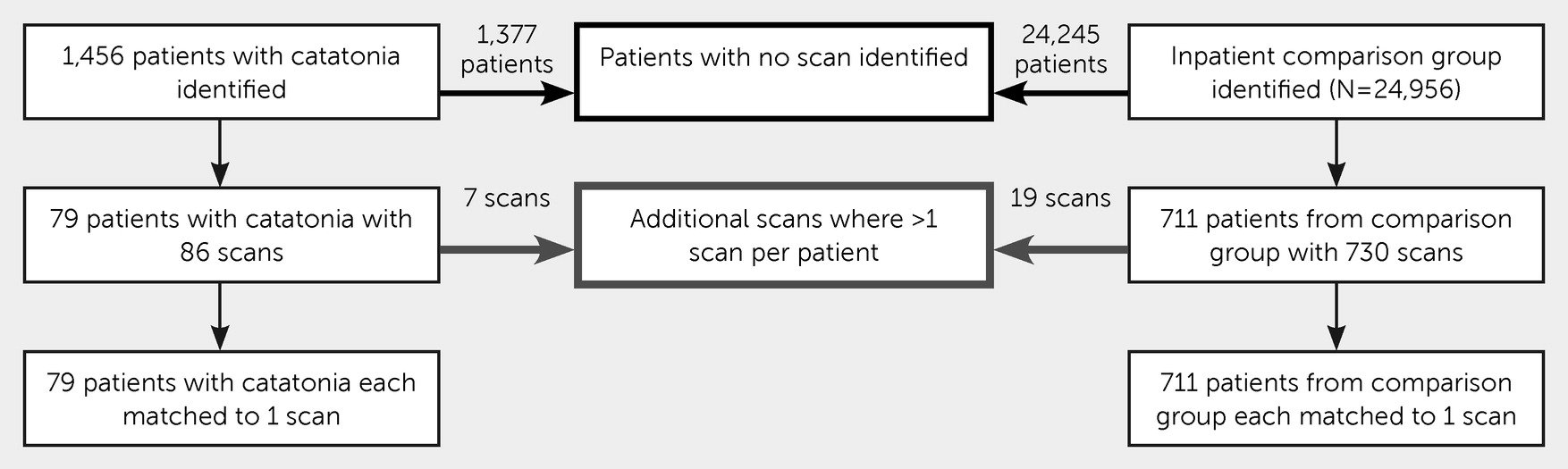
The exposure was an abnormal MRI scan, as judged by the reporting neuroradiologist. The clinical scanner was a 1.5 Tesla GE HDx, with scans collected for clinical reporting, including high-resolution T1-weighted, T2-weighted, and FLAIR sequences without contrast. The electronic health care records and MRI clinical radiological reports were extracted, where available, from the electronic records for all patients admitted to a hospital ward who met the selection criteria described above. The following data were extracted from structured fields in the records: age at index date, sex, race-ethnicity, involuntary detention within 2 weeks following the index date, and primary ICD-10 diagnosis. The index date for patients with catatonia was the date of the first identified catatonic episode; for the comparison group, the date of hospital admission was used as the index date. When a diagnosis had been made prior to the index date, the most recent diagnosis prior to the index date was used; when this was not available, the earliest diagnosis up to 6 months after the index date was used. MRI scans were reported by consultant neuroradiologists, of whom there are currently eight. Scans that occurred at any time before the index date or within 90 days after the index date were included. Scans obtained more than 90 days after the index date were excluded on pragmatic grounds, because there was a higher risk that they included abnormalities that had developed after the index illness. Where there were multiple scans available for one patient, the scan that was nearest to the index date was used. The procedure is illustrated in
Figure 1.
All available MRI reports from 2008 to 2018 were compiled in a spreadsheet. The reports were categorized and numerically coded for the presence of abnormalities by their anatomical location, pathological description, and lateralization. In scans with multiple abnormalities, each abnormality was coded separately by these criteria to minimize loss of data. Extracranial abnormalities were excluded. All reports were evaluated independently by two investigators (R.J. and R.T.), who were blinded to the diagnostic groups and each other’s assessments. Where there was disagreement, a third investigator (J.P.R.) arbitrated. The study size was determined pragmatically based on the number of available cases.
Following data collection, small cell sizes were merged based on a priori relationships between categories blind to group membership. The anatomical areas were merged based on embryological brain structure, and the pathologies were merged according to the main underlying mechanism.
Confounders
The potential confounders considered were age on date of scan, sex (male or female), Black race-ethnicity, and diagnostic group. We chose to adjust for these potential confounders because they have previously been associated with differences in brain MRI findings (
20–
24) and have been associated with risk of catatonia in prior studies (
25–
28). Ethnicity categories were grouped according to the preferred categories of the United Kingdom Office for National Statistics (
29). Mixed or multiple ethnic groups were combined with “other” ethnic group to avoid small cell sizes. Primary diagnoses were grouped as organic and neurodevelopmental disorders (
ICD-10 codes F00–F09, F70–89, F90, F95, and non-F codes), schizophrenia and related disorders (F20–F29), mood disorders (F30–F39), neurotic disorders (F40–F59), personality and behavioral disorders (F50–F69, F91–F94, and F98), and substance use disorders (F10–F19).
Statistical Analysis
We investigated whether having an abnormal MRI scan was associated with greater odds of reporting catatonia; we used univariable and multivariable logistic regression models adjusted for age, sex, and race-ethnicity.
Given the differing proportions of organic or neurodevelopmental diagnoses across the groups, we conducted a sensitivity analysis in which we excluded these diagnoses. As a secondary analysis, among the abnormal scans, we conducted a logistic regression for catatonia based on the number of abnormalities per scan, adjusted for age, sex, Black race-ethnicity, and diagnostic group.
We analyzed lateralization, anatomical location, and pathology by the number of scans that had at least one abnormality in the specified category. This was done to avoid scans with many abnormalities excessively weighting the analyses. To calculate the differences between proportions having different categories of abnormalities, we used Fisher’s exact test, because there were numerous small cell sizes.
Missing data were assumed to be missing at random. Therefore, to explore the impact of missing data on our estimates, as a sensitivity analysis, we imputed missing exposure data for participants with complete outcome data by using multiple imputation by chained equations. We imputed 20 data sets using all variables included in the models, as well as a number of auxiliary variables that were associated either with one of the variables of interest or with missingness of one of the variables of interest. The variables included in the final imputation model were abnormal scan, catatonia, age at scan, sex, Black race-ethnicity, diagnostic group, electroconvulsive therapy use within 2 weeks after index, age at index (either onset of catatonia or hospital admission), date of birth, date of scan, diastolic blood pressure, systolic blood pressure, date of death, time from referral to index date, time from index date to documentation, end date of catatonic episode, Health of the Nation Outcome Scale (HoNOS) score, HoNOS date, index date, duration of admission, Mini-Mental State Exam score, episode order, death within follow-up, involuntary detention, and validity of MRI report. (The observed and imputed data are compared in Table S1 in the
online supplement to this article.) The analysis used Stata MP, version 15.1.
This article was written according to the STROBE guidelines (
30). (The STROBE checklist is available in Table S2 in the
online supplement.)
Discussion
Neuroimaging abnormalities in patients with catatonia have previously been described in case reports and other studies with small sample sizes, often without a comparison group. This study used a large data set to describe common structural neuroimaging findings in patients with catatonia and compared these with findings for psychiatric patients without catatonia.
In terms of descriptive data, we found that MRI abnormalities were commonly reported in individuals with catatonia who had a scan, being present in 27 of 79 scans (34%). It was common for there to be more than one abnormality in each scan. Most abnormal scans had at least one abnormality reported that was bilateral (23 of 27), that affected the forebrain (25 of 27, of which 18 had a diffuse cerebral distribution), and that involved atrophy (17 of 27), although some of these scans also had other types of abnormalities reported. However, when we compared the scans between the groups with and without catatonia, we found no differences in the proportion of scans reported to have an abnormality, after adjustment for age, sex, Black race-ethnicity, and diagnostic group. Secondary analyses also found no evidence for a difference in the number of abnormalities, lateralization, anatomical location, or pathology.
To our knowledge, this is the largest study of catatonia neuroimaging published to date (
15). It also had the advantage of representing patients with catatonia across a range of underlying disorders, and it had an appropriate comparison group of psychiatric inpatients without catatonia.
However, there are a number of evident limitations, many inherent to the use of electronic health care records. The most important bias is related to the fact that our patients with neuroimaging were likely to be unrepresentative of all psychiatric inpatients because of the various reasons that they may have been referred for a scan. The reasons for ordering a scan were not available and are likely to differ between the catatonia and the comparison groups, and this would potentially lead to a selection bias. The characteristics of the comparison group have been shown to have a substantial effect on outcomes in studies of neuroimaging in psychiatric patients (
31). When a patient did not have an MRI scan, this was generally because it was not requested by the clinician. There is no consensus on whether many groups of psychiatric patients should undergo neuroimaging, but there is evidence that patients who are older and who are suspected to have organic diagnoses are more likely to be referred for neuroimaging (
32,
33).
In terms of missing data, on occasion, an MRI scan may have been performed in another hospital, it may have been performed outside the window for this study, or the patient’s lack of cooperation meant that no useful data could be extracted from the scan. Sex and race-ethnicity were occasionally missing (for 0.02% and 1.5% of patients, respectively) in the overall data set, and this was due to an absence of administrative coding of this information in the patient records. Although our sensitivity analysis using multiple imputation was likely to provide a more accurate estimate than complete-case analysis, the model was not able to include all the variables that would ideally be present to assert a missing-at-random hypothesis (such as the presence of focal neurological signs, pre-existing neurological disorders, seizures, or head injury) (
32), and thus it is likely that it was not a wholly adequate method of dealing with the missing data.
In terms of confounding, we were able to adjust our analysis for demographic variables, but there were likely to have been other relevant variables (such as cardiovascular risk factors or cognitive function) for which data were not available. Neuroradiologists sometimes reported findings differently and likely had different thresholds for what was worthy of mention. These reports may have been biased by the clinical information presented and the questions asked when the scan was requested. This may in part explain why the proportion of individuals with catatonia with an abnormal MRI scan is somewhat lower than in some previous smaller studies. Medda et al. (
34) described 26 patients with catatonia resistant to benzodiazepine treatment finding that the computed tomography (CT) or MRI scan was abnormal in 17 (65%, 95% CI=44%, 83%). Smith et al. (
28) examined the MRI scans of 31 patients with catatonia, finding abnormalities in at least 18 (58%, 95% CI=39%, 75%). It is possible that our study has provided a more conservative estimate, because its larger size means it was less susceptible to reporting bias.
There is, however, some consistency with other structural neuroimaging studies in terms of the type of abnormalities. Three other studies have shown extensive or generalized atrophy (or its proxy, enlarged cerebrospinal fluid spaces) as the most common neuroimaging abnormality (
28,
34,
35). A large number of case reports of focal lesions associated with catatonia have been reported, but most of these cases are of diffuse or multiple abnormalities (
15). Taken together, our findings support a weight of evidence that catatonia is associated with dysfunction of brain networks, rather than being the product of damage to isolated brain regions (
10). This is consistent with a quantitative study of MRI images that found reduced gray matter volumes in individuals with catatonia in areas within the frontothalamic and corticostriatal networks (
36).
However, when we examined the comparison to psychiatric patients without catatonia, we found no evidence of a difference in the proportion of abnormal scan reports after adjustment for demographic variables. To our knowledge, no prior studies have compared clinical neuroradiological reports of MRI scans in patients with catatonia and in a psychiatric comparison group. Two studies conducted this analysis using CT scan results, but one had just five patients with catatonia (
35), and the other focused solely on cerebellar atrophy (
37). These findings emphasize the high rate of brain abnormalities in patients with psychiatric disorders, especially schizophrenia and other neuropsychiatric conditions severe enough to require admission, and the need for a psychiatric comparison group in studies of catatonia. Previous work with data from the same center found that only 12.3% of MRI scans were abnormal; however, the mean age in that sample was 26 (compared with 44.5 for our comparison group), and all were under evaluation for first-episode psychosis (
38). It seems likely that the older age and greater disease severity of our comparison group led to the detection of more abnormalities, but it is notable that, even after the analysis adjusted for age, there was no evidence that individuals with catatonia were more likely to have an abnormal MRI scan. Adjustment or matching for factors such as psychopathology or neurological signs might be helpful.
Conclusions
Patients with catatonia commonly had MRI scan abnormalities reported, most frequently diffuse atrophy, but there was no evidence that such abnormalities occurred at a higher frequency among these patients, compared with other psychiatric inpatients. This finding is consistent with there being a basic neurological vulnerability to the condition, which relapses and remits, but which may be specifically driven by metabolic or physiological dysfunction. Researchers should consider the benefits of using large clinical samples to study patients with relatively rare and hard-to-recruit conditions, such as catatonia, while mitigating the lack of systematic detail inherent in the qualitative neuroradiological evaluation of clinical MRI scans. However, use of routine health care records has notable limitations, including heterogeneous control groups, selection bias, and varying reporting thresholds from radiologists. Quantitative volumetric analysis or functional neuroimaging techniques, such as arterial spin labeling, in operationally defined cases and a comparison group chosen to minimize selection bias remains the ideal research design, and longitudinal studies assessing the stability of neuroimaging abnormalities in catatonia will also be important.