Emotional reactions have been defined as short-lived bursts of psychological-physiological coordination to adapt to changing environmental demands or stimuli (
1). For example, when suddenly faced with a large snarling dog, most people will open their eyes wide and pull out the corners of their lips in an expression of fear, experience increased heart and respiration rates, and describe themselves as feeling frightened. Because the coordination among these aspects of emotion is often thought to be normative (
2,
3), in everyday social interactions people tend to assume that the exterior expression of emotion by others represents that person’s subjective internal experience as well.
These various aspects of emotion, however, are not always linked. For example, disconnected or incongruent states may result intentionally during deception or play-acting. Neurological diseases can also cause unintentional incongruences between aspects of emotion. Studies involving patients with Parkinson’s disease, for example, have demonstrated that degeneration of subcortical pathways results in loss of spontaneous emotional expression, perhaps with relative preservation of deliberate facial expression (
4). Pseudobulbar affect involves the opposite: hyperemotional expression without accompanying subjective self-report of emotional content (
5). Such incongruences can cause social embarrassment, misunderstanding, and distress (
6–
9).
The objective of this study was to compare incongruences between facial expression and self-reported emotional reactivity among individuals with several neurodegenerative diseases, with a focus on frontotemporal dementia (FTD). FTD comprises a group of three main syndromes: the behavioral variant (bvFTD), the nonfluent variant of primary progressive aphasia (nfvPPA), and the semantic variant of primary progressive aphasia (svPPA). Another variant, the right temporal variant of primary progressive aphasia, or right temporal FTD (rtFTD), has also been described as overlapping between semantic dementia and bvFTD (
10). Whereas bvFTD and rtFTD are primarily disorders of social and emotional dysfunction, svPPA and nfvPPA are primarily disorders of language (
11,
12). In addition, we explored two parkinsonian syndromes that are frequently comorbid with FTD. Progressive supranuclear palsy (PSP) is a parkinsonian disorder that sometimes overlaps with FTD syndromes and pseudobulbar affect (
13). Corticobasal syndrome (CBS) is associated with a variety of underlying pathologies but may also correlate with parkinsonism and FTD syndromes (
14). Finally, we also investigated Alzheimer’s disease (AD), the most common form of degenerative dementia (
15).
Whether due to parkinsonism, lack of emotional insight, diminished linguistic ability, or other reasons, neurodegenerative diseases offer several possible reasons for incongruent facial expression and self-reported emotional experience. Diminished reactivity has been described previously related to emotions such as sadness (
16) and disgust (
17). Another study compared multiple aspects of emotional reactivity and demonstrated more subjective reporting of “nontarget” emotions (i.e., the extent to which someone reports experiencing positive emotion to a negative stimulus or vice versa) than a healthy control group (
18). Our primary hypothesis was that, despite wide ranges of incongruences between facial expression and self-reported emotional reactivity, especially related to bvFTD’s known phenotypical, genetic, and histopathological heterogeneity (
19), many individuals with neurodegenerative diseases would differ from a healthy control group in the extent to which spontaneous facial expression corresponds to self-reported spontaneous emotional experience.
For each group that differed significantly from a healthy control group in the extent to which spontaneous facial expression differed from self-reported emotional experience (FE-SR), we aimed to explore additional factors that could influence FE-SR. We first investigated the two components of the difference itself: facial expression and self-reported emotional experience. We then further explored physiological activity (measured with a composite score of autonomic measures associated with emotional response), parkinsonism (assessed by using the Unified Parkinson’s Disease Rating Scale–III [UPDRS-III]) (
20), and a nontarget response self-report score (measured as the extent to which someone reported experiencing positive emotion to a negative stimulus and vice versa). The nontarget response score quantifies, through self-report, the experience of nontarget emotions, which have previously been shown to have negative consequences for caregivers (
21). Nontarget response may be due to a diminished ability to interpret interoceptive information (as first suggested by James [
22]), which is implicated in bvFTD as a result of insular involvement (
23–
25); due to decreased ability to put experiences into words, as may be seen in the case of primary progressive aphasias related to language deficits; and perhaps, with bvFTD, due to impulsive and disinhibited verbal responses contrary to their actual internal state (
18).
Methods
Participants
A healthy control group (N=36) and patients with a neurodegenerative disease (AD, bvFTD, rtFTD, svPPA, nfvPPA, CBS, or PSP) (N=89) were selected from a data repository of individuals who had completed the main tasks of interest (i.e., watching three film clips chose to elicit emotion) between July 2004 and December 2012. Patients were first seen and diagnosed at the University of California, San Francisco (UCSF), where they underwent extensive evaluation, including neuropsychological testing, neurological examination, and imaging studies. The participants were then seen at the University of California, Berkeley (UC Berkeley), where they received a comprehensive assessment of emotional functioning (
26). The institutional review boards of UCSF and UC Berkeley both approved the study, and informed consent was obtained for all participants.
The diagnosis of AD was established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders criteria, and included amnestic, dysexecutive, and behavioral subtypes (
27). Corticobasal degeneration was established by using UCSF Memory and Aging Center criteria from 2003. PSP diagnoses were established by using criteria from Litvan et al. (
28), and primary progressive aphasias (svPPA and nfvPPA) were diagnosed by using criteria outlined in Gorno Tempini et al. (
11). Thirteen participants were diagnosed as having rtFTD by a panel of neurologists, neuropsychologists, and speech and language specialists by using available examination and structural MRI data (
21). Those with bvFTD were diagnosed initially by using criteria from Neary et al. (
29) and were required to meet 2011 international criteria for inclusion in the study (
12).
Film Clips to Elicit Emotional Reaction
Participants watched three short films, each chosen to elicit a different emotion. The films shown were an excerpt from the television show I Love Lucy in which two women wrap chocolates, chosen to elicit amusement; an excerpt from the movie The Champ in which a boy cries after his father dies, chosen to elicit sadness; and an excerpt from the television show Fear Factor in which a man eats cow parts, chosen to elicit disgust. These film clips were chosen because of their demonstrated capability to induce strong emotional responses in healthy adults.
Primary Outcome
Our primary outcome was a difference score between facial expression and self-reported emotional reactivity (i.e., FE-SR). We used a difference score because we wanted to focus on differences between spontaneous facial expression and self-reported emotional experience for each individual, rather than across entire groups, as would be done by means of a statistical interaction. A positive score would indicate more appropriate facial expression than appropriate self-reported emotional reactivity (e.g., someone who smiled a lot during an amusing video but who nevertheless reported feeling little emotion). A negative score would indicate less appropriate facial expression than appropriate self-reported emotional reactivity (e.g., someone who frowned or made little facial expression when watching an amusing video, but who nevertheless said it was very funny).
Self-Reported Reactivity
After each video, participants self-reported how intensely they felt each of a list of emotions during the film clip (i.e., affection, fear, amusement, anger, shame, disgust, embarrassment, enthusiasm, pride, or sadness) by using a scale with scores of 0, not at all; 1, a little; and 2, a lot. Responses were then grouped by positive or negative emotions, and composite scores were calculated for each individual by summing the original scores for both positive and negative self-reported emotions after each video. “Appropriate” responses (e.g., negative scores for a sad film) were then z-scored to bring all measures onto the same scale and were again summed across target emotion types for inclusion in the overall difference score.
Facially Expressed Reactivity
Participants were videotaped with a partially concealed video camera placed behind darkened glass on a bookshelf. Facial expression during a 30-second segment of each film was later coded, second by second, by trained coders by using the Emotional Expressive Behavior Coding System (
30,
31). Coders were blind to diagnosis, research hypotheses, and the content of the films the participants were watching. Ten responses—anger, contempt, confusion, disgust, fear, happiness/amusement, embarrassment, interest, sadness, and surprise—were coded on a scale rating the intensity of emotion from 0 to 3. Intercoder reliability was high (intraclass correlation coefficient=0.76). Scores were normalized and summed into two composite scores for negative and positive facially expressed reactivity for each task. Appropriate responses were then z-scored to bring all measures onto the same scale and were summed for inclusion in the difference score.
Secondary Measures
Secondary measures were obtained to explore potential explanations for found differences between groups in facial expression or self-reported emotional reactivity. For example, diminished facial expression with intact self-reported emotional reactivity could be due to a movement disorder such as parkinsonism, which is common in FTD (
32,
33). Alternatively, individuals with diminished autonomic reactivity despite intact self-report (e.g., alexithymia or poor emotional insight), may have different autonomic measures from other groups. A low difference score may result from nontarget self-reporting. For example, if someone self-reported that they felt all of the listed emotions “a lot,” regardless of whether the emotions were positive or negative (as might be seen for someone with a semantic dementia or with perseveration or disinhibition from any cause), they would still be assessed as self-reporting an appropriate emotion at least part of the time, even though this self-report would not necessarily reflect their true subjective internal state.
Motor Disorders
All patients who participated underwent a detailed examination, including assessment with the UPDRS-III (
20). This well-validated and standardized assessment of parkinsonism is widely used in studies of Parkinson’s disease and other movement disorders, such as PSP and CBS, but has also been increasingly investigated in studies of FTD (
32).
Physiological Activation
Sensors were applied to each participant’s chest, nondominant hand, and right ear to measure physiological reactivity with a system that included a polygraph test and a computer. Physiological measures included interbeat interval, calculated as the time between successive R waves by using electrodes placed on opposite sides of the participant’s chest; finger pulse amplitude, recorded via a photocell attached to the index finger of the participant’s nondominant hand; finger pulse transmission time, calculated as the difference in time from the electrocardiogram (EKG) R wave and upstroke of pulse at the finger; ear pulse transmission time, calculated as the difference in time between the EKG R wave and the upstroke of pulse at the ear; skin conductance level, measured as a constant-voltage device’s signal between electrodes on the ring and index fingers of the participant’s nondominant hand; and general bodily activity, assessed via an electromechanical transducer attached to a platform under the participant’s chair. The recorded measures were adjusted for a physiological baseline recorded before emotional stimulation and were then compiled into a composite score to reflect physiological arousal for each individual during each presented stimulus.
Nontarget Verbal Response
We considered the possibility that some participants were answering similarly but meaninglessly to all questions about emotional stimuli (i.e., responding “a lot” to all questions asked), as could be the case for participants with disinhibition or semantic loss. This response could theoretically increase the gap between participants’ facial expression and their self-reported emotional reactivity but not necessarily reflect their internal emotional experience accurately. To address this possibility, we summed the self-report scores that represented an “inappropriate” valence to each of the three presented emotion-provoking stimuli (e.g., a positive emotional self-report to a negative stimulus) as a proxy for such nontarget answering or nontarget responses.
Neuropsychological Measures
To confirm expected patterns of cognitive deficits, participants with neurodegenerative disease completed a comprehensive battery of neuropsychological tests at the UCSF Memory and Aging Center. Selected and relevant measurements included a forward and backward digit span to assess working memory (
34), phonemic and category verbal fluency tasks, the nine-item California Verbal Learning Task (
35), a modified Rey-Osterrieth Complex Figure (
36), and the 15-item Boston Naming Test (
37). The Interpersonal Reactivity Index and Neuropsychiatric Inventory were administered to the caregiver to report on the participant (
38). Geriatric Depression Scale, Mini-Mental State Examination, and Clinical Dementia Rating scores were also obtained (
39–
41).
Statistical Analyses
In all analyses, the omnibus multivariate protective F test was used in multiple regression, allowing for separate variances per diagnostic group. We first compared the FE-SR difference score between groups, with an a priori p<0.05. We also investigated the absolute value of FE-SR to explore FE-SR differences regardless of directionality (i.e., whether facial expression was less or more than self-reported emotion) and the difference in confidence intervals to investigate whether the diagnostic groups also differed from a healthy control group in the amount of individual variability of the difference score. We also explored between-group differences in FE-SR regarding each of three emotion types: amusement, disgust, and sadness. Age, gender, and education were included as covariates.
Although our design was not optimal (i.e., all measures were obtained at essentially the same time), and we were not adequately powered for true mediation analysis, we nevertheless performed exploratory analyses on potential explanatory variables in order to consider their potential as explanations for between-group differences in the FE-SR difference score. Additional analyses (i.e., components of the difference score, UPDRS-III, physiological reactivity, and nontarget response) focused on groups that differed from the healthy control group. Because these additional analyses were considered exploratory, we did not adjust for multiple comparisons. The exploratory analyses were done by comparing the center values of the measure in the diagnostic groups and healthy control group, examining the correlation between the difference score and the measure of interest within the diagnostic group, and determining whether there was a significant interaction between the slopes in the correlation between the difference score and each candidate variable in the group with neurogenerative disease and the healthy control group. For example, if the bvFTD group were found to differ from the healthy control group in FE-SR and if this difference were due to diminished facial reactivity explained by parkinsonism, we might expect that UPDRS-III scores would differ between the bvFTD and healthy control groups, UPDRS-III scores would correlate with FE-SR in the bvFTD group, and/or the relationship between UPDRS-III score and FE-SR would be stronger among those in the bvFTD group than in the healthy control group.
Results
Comparison of Behavioral Measures of Interest
Participants’ demographic characteristics and neuropsychological test profiles are summarized in
Table 1. Participants with bvFTD (
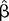
=−2.32, p=0.038, t=−2.47, 95% confidence interval [CI]=−4.49, −0.14), svPPA (
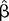
=−2.28, p=0.010, t=−3.44, 95% CI=−3.96, −0.60), or nfvPPA (
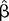
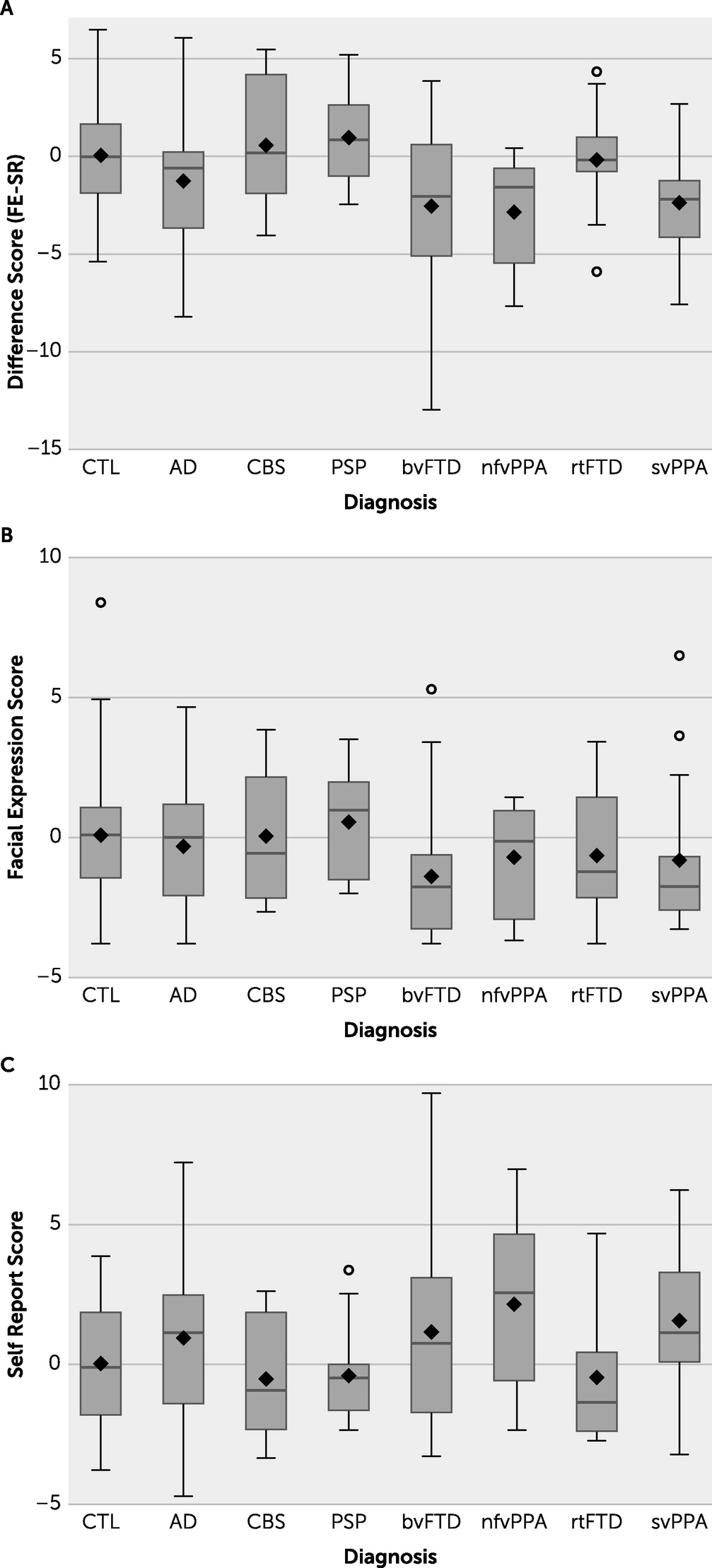
=−3.14, p=0.007, t=−3.24, 95% CI=−5.24, −1.04) all differed significantly from the healthy control group in the extent to which self-reported emotion was congruent with facial expression (i.e., FE-SR) (
Figure 1). Specifically, compared with the healthy control group, participants with each of the three FTD variants expressed less emotion on their faces than they reported verbally. The protective F test was statistically significant (p=0.013). When we investigated the absolute value of FE-SR (i.e., the magnitude of the difference without any sense of directionality), no diagnostic group differed from the healthy control group at the level of statistical significance except for bvFTD (
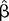
=1.7, p=0.035, T=2.26, 95% CI=0.14, 3.3). The standard deviation around the mean for facial reactivity in the bvFTD group was the largest of any diagnostic group and was significantly larger than for the healthy control group (standard F test for unequal variances bvFTD vs. healthy control group, p=0.004).
We also explored between-group differences in FE-SR for each of the three emotion types: amusement, disgust, and sadness. Regarding amusement, the AD group (
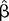
=0.012, p=0.012, CI =−1.8, −0.2) and svPPA group (
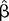
=−1.4, p=0.002, CI=−2.2, −0.6) differed from the control group. Regarding disgust, FE-SR for the nfvPPA group (
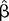
=−1.6, p=0.012, CI=−2.8, −0.4) significantly differed from the control group. The nfvPPA group also differed from the control group regarding sadness (
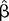
=−1.0, p=0.019, CI=1.90, −0.20), as did the bvFTD group (
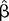
=−1.1, p=0.029, CI=−1.99, −0.11). In analyses using absolute values, no groups differed from the control group in disgust or sadness, although the svPPA group again differed significantly from the control group in amusement (
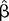
=0.66, p<0.11, CI=0.17, 1.16).
Exploratory Analyses
To understand why the bvFTD, svPPA, and nfvPPA groups expressed less emotion facially than verbally, we first explored the two components of the difference scores: facial reactivity and self-reported emotional reactivity. No significant differences in facial reactivity and no statistically significant differences involving participants with svPPA or nfvPPA were found.
We next considered three potential contributing factors for each group difference, including parkinsonism (measured via the UPDRS-III), physiological reactivity (measured with a composite score), and a measure of nontarget answering. To gauge the potential for a mediation, we considered differences between groups, the interaction between groups, and the relationship between each factor and the primary outcome variable for each of the three potential contributing factors. However, we were underpowered for a true mediation analysis. The results of these analyses are summarized in
Table 2 and in Tables S1 and S2 in the
online supplement to this article. (For differences between groups and for the relationship between exploratory and main variables within each group, see Figure S1 in the
online supplement; for potential interaction between groups, see Figure S2 in the
online supplement.) A potential mediator would ideally have statistically significant relationships in each table. However, none of the potential explanatory variables met that condition.
Although parkinsonism was higher among participants with bvFTD than those in the healthy control group (
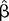 =
=4.63, p=0.030, 95% CI=0.51, 8.75), this difference did not fulfill criteria for further mediating FE-SR. Moreover, there was a statistically significant correlation between autonomic reactivity and the difference score in the bvFTD group (
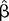
=1.50, p=0.039, 95% CI=0.08, 2.91), and the effect was statistically significantly different from that of the healthy control group (interaction:
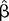
1.64, p=0.033, 95% CI=0.15, 3.13). The autonomic response was associated with a greater facial response in the bvFTD group (
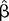 =
=1.08, p=0.004, 95% CI=0.41, 1.75), and this relationship seemed stronger than in the healthy control group (interaction:
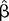
0.97, p=0.015, 95% CI=0.20, 1.73; F test for association between autonomic reactivity and facial expression, p=0.016).
Among participants with bvFTD, there was a marginally greater (although statistically nonsignificant) tendency to provide nontarget responses compared with the healthy control group, and there was a statistically significant relationship between nontarget answers and the difference score (
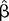
−0.65, p=0.004, 95% CI=−1.05, −0.25). Again, there was no evidence of interaction between group and nontarget answering regarding the difference score outcome. The protective F tests found a statistically significant difference in the effect of nontarget answering on the difference score outcome (p=0.011) but no statistically significant effect modifier by diagnosis (p=0.147) and no statistically significant mean differences among diagnostic groups (p=0.192).
There was a marginally greater (although statistically nonsignificant) tendency for those with svPPA to provide nontarget responses compared with the healthy control group, which may support the notion that differences were driven by semantic loss for labeling of emotion in self-report. However, there was no statistically significant relationship between providing nontarget responses and the overall difference score, nor was there a statistically significant interaction between diagnostic group and providing nontarget responses.
To summarize, our exploratory analyses did not demonstrate any potential mediators of the overall effect described (i.e., did not explain why individuals with all well-defined variants of FTD appear to have less facially expressed emotion than self-reported emotion).
Discussion
We hypothesized that patients with different neurodegenerative conditions would differ in emotional congruence, defined as the extent to which their self-reported emotion matched their facially expressed emotion. We found that groups representing the three main subtypes of FTD all showed lower emotional congruence, as indicated by expressing less facial emotion than self-reported emotion, compared with a healthy control group. When we investigated the absolute value of FE-SR difference, regardless of directionality of the difference score, bvFTD was the only group that differed from the healthy control group.
We also investigated FE-SR individually for each of the three included emotional stimuli. The most robust difference was for the svPPA group when viewing the film chosen to elicit amusement. We speculate that cultural nuance and appropriateness in amusement likely drive the disconnection in amusement for those with svPPA. The nfvPPA group also differed from the control group in FE-SR related to amusement, which may be driven by nfvPPA having some stereotyped facial expressions associated with disgust and sadness, such as a knit brow, which would have been read as inappropriate valence by the coding system. Although we suspect differences in FE-SR in response to the sadness stimulus were primarily driven by empathetic loss among those with bv-FTD, the data overall suggest high amounts of heterogeneity in this population; a larger sample would be needed to further explore the phenomenon among subtypes because etiologies likely differ significantly between individuals.
We explored potential explanations for overall differences in FE-SR, but none of the potential explanations we explored (i.e., nontarget answering, parkinsonism, or physiological activity) met all the criteria required of a potential mediator. Nevertheless, our results demonstrate a range in the extent of disconnections between different aspects of emotional experience among patients with FTD. Moreover, these findings may have important implications regarding how clinicians and caregivers interpret both verbal and nonverbal signals of emotion by these patients.
Lower Coherence in Facial Expression and Self-Report
Participants with the three most established forms of FTD (i.e., bvFTD, nfvPPA, and svPPA) all displayed lower FE-SR congruence, as indicated by less facial reactivity than self-reported emotion, compared with a healthy control group. The absolute value of the difference score appeared to be greatest in the bvFTD group, suggesting that the difference between facially expressed and self-reported emotion may be especially marked among participants with this diagnosis. In addition, the confidence intervals of FE-SR were broadest for the bvFTD group, suggesting not only that individuals with bvFTD generally differ from healthy people in the extent to which facial expressions are congruent with self-reported emotion but also that the extent of incongruence may vary especially widely in this group. This suggestion corresponds with the increasing recognition that bvFTD is a heterogeneous disorder with perhaps four different clusters of neurodegeneration (
19) and many different underlying histopathologies (
42). In contrast, the semantic and nonfluent variants of PPA, which did not show this elevated variability, are more neuroanatomically and histopathologically predictable than bvFTD (
42).
Exploratory Analyses
The remainder of our analyses were exploratory and failed to demonstrate any clear potential mediator of incongruences between facial expression and self-reported emotional reactivity. It remains possible that some patients with bvFTD may have a blunted affect, similar to that described previously in schizophrenia (
43), in which patients have been found to move their faces less than their self-reported emotions would suggest appropriate (
44). Authors describing this phenomenon in schizophrenia have urged caution about dismissing self-reported emotion among patients who do not display that emotion on their faces, and this caution may also be appropriate regarding those with bvFTD. Although UPDRS-III scores did not correlate well with the facial expressivity outcome, we separately reviewed at least one case where facial expression of an individual with FTD was selectively altered in a manner more akin to an apraxia (
45). With a larger sample, neuroimaging studies, and perhaps further subdividing of FTD subtypes, we might be able to predict altered facial expression in atrophy patterns associated with the anterior-superior insula, although there are also reasons to think that the supplementary motor area could be involved without relying on parkinsonism as a mediator (
46,
47). However, the correlation between autonomic reactivity and facial regulation in bvFTD suggests that part of the decreased facial expression relative to self-reported emotion may be related to diminished autonomic reactivity in bvFTD, which is associated with atrophy in the ventral anterior insula and anterior cingulate cortex. Diminished autonomic reactivity has been suggested in prior studies but was not seen in our study population (overall F test, p=0.276). Use of high-speed videography or facial electromyography combined with physiological measurements may permit further disentanglement of these emotional components (
48).
Another possible explanation for increased FE-SR is alexithymia: patients with bvFTD may have diminished insight into their own emotions (
49). This possibility was approximated here by nontarget answering, but alexithymia is a complex variable that may also relate more to impulsivity, language difficulties (as suspected here in svPPA or nfvPPA), or other aspects of executive dysfunction. Poor internal representation of an emotional response could also cause diminished autonomic reactivity, facial expression, and self-report, although we might then expect more consistent correlations between all of these various emotional facets. The breadth of the confidence interval suggests that bvFTD is likely too heterogeneous to allow easy detection of one common mechanism underlying incongruence between facially expressed and self-reported emotional reactivity, even though the absolute magnitude of the effect may be larger in bvFTD than in other forms of dementia. Pairing these tests with detailed tests of linguistic ability and exploring further subsamples from larger cohorts may further elucidate the role of nontarget reporting in mediating greater FE-SR in all forms of FTD.
In contrast to bvFTD, participants with svPPA or nfvPPA were predicted to have problems with verbal report of emotion, but this again was not found to be the case in our analyses. Additionally, even though UPDRS-III scores were higher in some patient groups than others and prior publications had described the effects of neurodegeneration on emotional displays (
4,
45), these scores had no impact on the FE-SR difference score.
The exploratory analysis revealed some intriguing associations, such as a correlation between stronger facial responses and stronger autonomic responses in the bvFTD group. A weak physiological response, then, could be associated with a weak facial response, contributing to a more negative difference score (e.g., less facial expression than self-reported activity). Stronger physiological responses may be associated with a stronger facial response, contributing to a more positive difference score (e.g., more facial movement than self-reported emotional reactivity), as was noted. Significant changes in autonomic function (a major component of our measure of physiological reactivity) have been described previously in bvFTD (
50–
53). Once again, however, these relationships failed to meet all three of our requirements to be considered a potential mediator of incongruence between facial expression and self-reported emotion, and any further extrapolation must be treated with caution.
Strengths and Limitations
Whereas other studies have previously explored both facial expression and self-reported emotion among individuals with FTD, analyzing these measures separately, we focused on the extent to which the coherence between these two measures of emotional expression differed between FTD subtypes and a healthy control group. We accomplished this goal by using techniques that focus on the difference between spontaneous facial expression and self-reported emotion in each individual rather than correlating across groups, as in previous studies. This approach allowed us to explore the range of discrepancy among individuals with each disorder. Indeed, our results showed a broad range of individual variability in these measures, suggesting that a larger sample would be useful in further exploring the causes of incongruent self-report and facial expression among individuals with these rare diseases. The presence of that variability, however, is an important finding, demonstrating the need for caution when applying knowledge gained from group-based research on FTD patients’ emotional states to individual patients. For example, rather than simply stating that a patient with bvFTD whose facial expressions do not match his or her self-reported emotional state lacks insight, a broad range of other possible explanations should also be considered. What is true for one bvFTD patient, or even for most bvFTD patients, will not be true for all. Because we aimed to focus on the discrepancy between facial expression and self-reported emotion broadly, we performed exploratory analyses of possible causes of this discrepancy across all groups of emotion. Future studies could further explore possible causes of discrepancies in specific emotions, such as amusement compared with sadness or disgust. These issues should be explored further in larger studies. Future studies could also allow for more levels of emotional arousal. Follow-up studies would also ideally include larger samples in order to explore other variables, such as nontarget facial expression and correlations between exploratory variables such as autonomic reactivity and nontarget responses, because both could affect self-report and diminished interoception could affect both self-report and facial expression.
Conclusions
Individuals with the three most widely recognized forms of FTD, including bvFTD, svPPA, and nfvPPA, showed reduced emotional coherence, in the form of less facial expression than verbal expression of emotion, compared with a healthy control group in a film-viewing task designed to elicit emotion. The absolute difference was greatest in the bvFTD group, and individuals with this disease also demonstrated the greatest variability in emotional coherence. We concluded that the potential for reduced emotional coherence among those with neurodegenerative diseases indicates a need for caution when attempting to infer a patient’s internal emotional state from verbal statements or nonverbal facial expressions.