Maternal, Fetal, and Child Outcomes of Mental Health Treatments in Women: A Meta‐Analysis of Pharmacotherapy
Abstract
Objective
Methods
Results
Conclusions
Highlights
METHODS
Scope of the Review

Data Sources and Searches
Inclusion and Exclusion Criteria
Study Selection
Data Extraction and Quality Assessment
Data Synthesis and Analysis
RESULTS
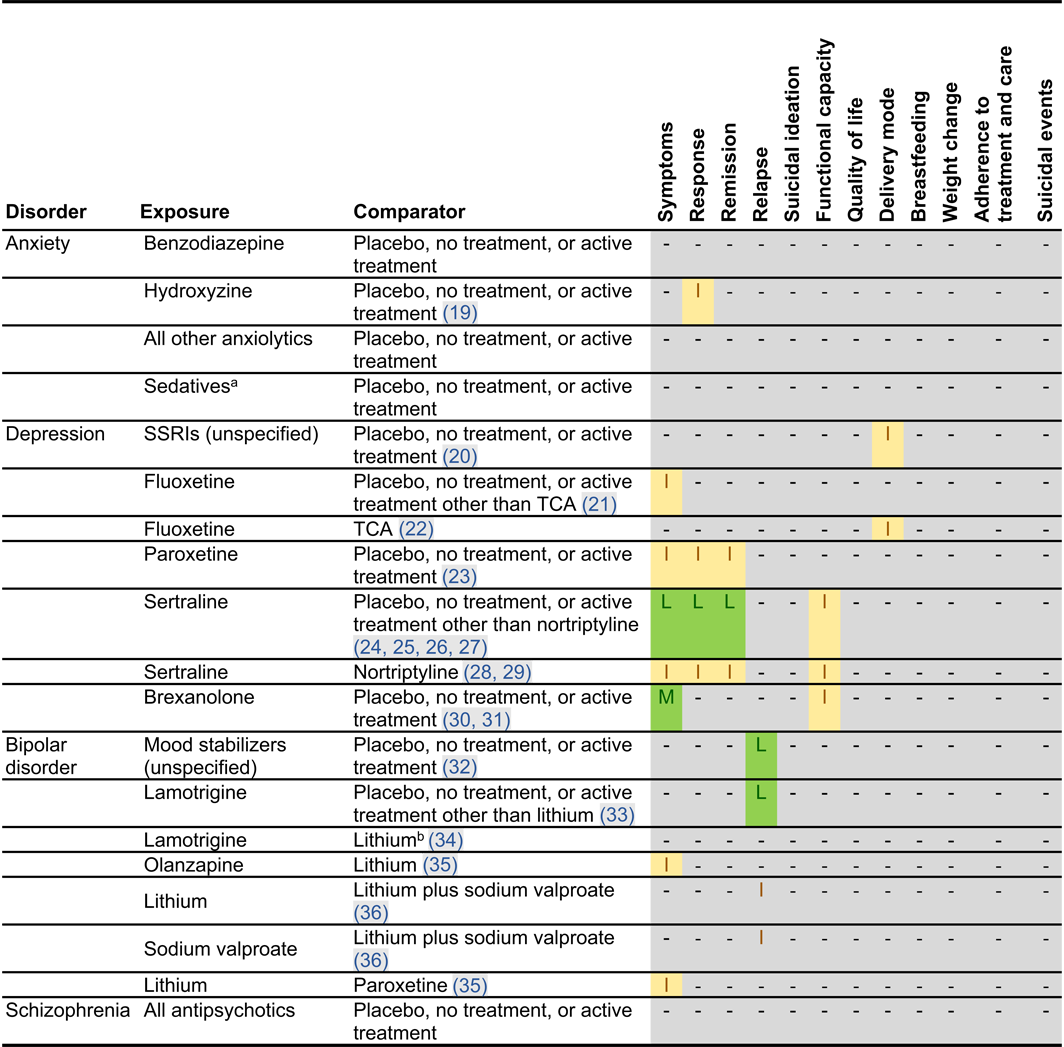
Effectiveness of Perinatal Pharmacotherapy
| Population | Outcomea | Exposure | Comparator | Incidence | N | Results | ARD per 1000 womenb (95% CI) | NNTB/NNTHc (95% CI) | Key considerations |
|---|---|---|---|---|---|---|---|---|---|
| Women with postpartum depression | Response | Sertraline | Placebo | 10/17 (59%) versus 5/19 (26%) | 36 | RR in low risk‐of‐bias study: 2.24 (95% CI, 0.95 to 5.24) (24) no difference between two arms in high risk‐of‐bias study, p = 0.054 (25) | 326 (11 to 1116) | NNTB:4 (1 to 91) | RCT evidence, small sample, imprecise results |
| Women with postpartum depression | Remission | Sertraline | Placebo | 9/17 (53%) versus 4/19 (21%) | 36 | RR: 2.52 (95% CI, 0.94 to 6.70); (24) no difference between two arms in high risk‐of‐bias study, p = 0.372 (25) | 320 (4 to 619) | NNTB:4 (2 to 250) | RCT evidence, small sample, imprecise results |
| Women with bipolar disorder | Recurrence from discontinuation | Discontinuedmood stabilizers | Continued mood stabilizers | 53/62 (85.5%) versus.10/27 (37%) | 89 | AHR: 2.2 (95% CI, 1.2 to 4.2) (32) | 268 (56 to 486) | NNTH: 4 (3 to 18) | Observational evidence, small sample, imprecise results |
| Women with bipolar disorder | Time‐to‐25%‐recurrence from discontinuation | Discontinued mood stabilizers | Continued lamotrigine | 16/16 (100%) versus 3/10 (30%) | 26 | AHR: 12.1 (95% CI, 1.6 to 91.7) (33) | 687 (135 to 700) | NNTH: 2 (2 to 8) | Observational evidence, study has limitations, small sample, imprecise results |
| Women with at least one anxiety diagnosis in the year before conception | Ectopic pregnancy | Benzodiazepine exposure 90 days before conception | No benzodiazepine exposure before conception | 249/9188 (2.71%) versus 1730/81,291 (2.13%) | 90,479 | ARR: 1.33 (95% CI, 1.17 to 1.51) (37) | 7 (4 to 11) | NNTH: 143 (91 to 250) | Observational evidence, potential for residual confounding |
| Women with mood or anxiety disorder | Postpartum hemorrhage | Exposed to SSRIs during delivery | Unexposed to SSRIs during delivery | 503/12,710 (3.96%) versus 1896/69,044 (2.75%) | 81,754 | ARR, 1.47 (95% CI, 1.33 to 1.62) (38) | 13 (9 to 17) | NNTH: 77 (59 to 112) | Observational evidence, potential for residual confounding |
| Women with mood or anxiety disorder | Postpartum hemorrhage | Exposed to SNRIs during delivery | Unexposed to SNRIs during delivery | 35/702 (5.0%) versus 1896/69,044 (2.75%) | 69,746 | ARR, 1.90 (1.37 to 2.63) (38) | 25 (10 to 45) | NNTH: 40 (23 to 100) | Observational evidence, potential for residual confounding |
| SNRI exposure or depression diagnosis, through second trimester | Preeclampsia | SNRIs exposure through second trimester | Unexposed depressed | 107/1216 (9%) versus 3215/59,219 (5%) (39); 23/408 (5.6%) versus 1569/65,392 (2.4%) (40) | 65,800 | ARR, 1.52 (95% CI, 1.25 to 1.83) (39); ARR, 1.59 (95% CI, 1.26 to 3.03) (40) | 14 (6 to 49) | NNTH: 72 (21 to 167) | Observational evidence, potential for residual confounding |
| Women with depression | Preeclampsia | Exposed to TCAs in pregnancy | Unexposed to TCAs in pregnancy | 47/441 (10.7%) versus, 3215//59,219 (5.4%) (39); 14/146 (9.59%) versus 1569/65,392 (2.40%) (40) | 65,538 | ARR, 1.62 (95% CI, 1.23 to 2.12) (39); ARR, 3.23 (95% CI, 1.87 to 5.59) (40) | 54 (21 to 110) | NNTH: 19 (10 to 48) | Observational evidence, potential for residual confounding |
| Women prescribed second‐generation antipsychotic | Gestational diabetes | Quetiapine continued in pregnancy | Quetiapine discontinued in pregnancy | 110/1543 (7.1%) versus 122/2990 (4.1%) | 4533 | ARR, 1.28 (95% CI, 1.01 to 1.62) (41) | 11 (<1 to 25) | NNTH: 88 (40 to 2439) | Observational evidence, potential for residual confounding |
| Women prescribed second‐generation antipsychotic | Gestational diabetes | Olanzapine continued in pregnancy | Olanzapine discontinued in pregnancy | 46/384 (12.0%) versus 49/1041 (4.7%) | 1425 | ARR, 1.61 (95% CI, 1.13 to 2.29) (41) | 29 (6 to 61) | NNTH: 35 (17 to 167) | Observational evidence, potential for residual confounding |
| Pregnant women with depression or anxiety | Spontaneous abortion | Benzodiazepine exposure in first trimester (42) or within the first 19 weeks (43) versus untreated or a history of mood disorders or anxiety during pregnancy | Unmedicated mental illness | 386/2384 (16%) versus 442/3647 (12%) (42) | 6031 | ARR, 1.6 (95% CI, 1.3 to 1.9) (42) | 73 (36 to 109) | NNTH: 14 (10 to 28) | Observational evidence, potential for residual confounding |
| 198 cases/570 controls versus 3221 cases/15 382 controls (43) | AOR: 2.85 (95% CI, 1.72 to 4.72) (43) | ||||||||
| Women with SNRI exposure or depression diagnosis in past 4 years | Spontaneous abortion | SNRI exposure in 1st trimester | Unexposed with depression diagnosis in past 4 years | 20/90 (22%) versus 720/7034 (10%); results corrected for induced abortions: 20/137 (15%) versus 720/8877 (8.1%) | 9014 | ARR, 2.1 (95% CI, 1.4 to 3.0); corrected for induced abortions ARR, 1.7 (95% CI, 1.2 to 2.6) (44) | 62 (16 to 130) | NNTH: 17 (8 to 63) | Observational evidence, potential for residual confounding |
| Women with a mental health disorder | NICU admission | Benzodiazepine exposure during pregnancy | Unexposed to benzodiazepine during pregnancy | 32/144 (22.2%) versus 125/649 (19.3%) | 793 | AOR, 2.02 (95% CI, 1.11 to 3.66) (45) | 133 (17 to 274) | NNTH: 8 (4 to 59) | Observational evidence, potential for residual confounding, benzodiazepine exposure may result in NICU admission by policy |
| Women exposed to SSRIs or unexposed with a psychiatric diagnosis | Apgar score <7 at 5 min | Exposed to SSRIs during pregnancy | Exposed to SSRIs before pregnancy or unexposed with a psychiatric diagnosis | 28/2664 (1.1%) versus 31/5141 (0.6%) (46) | 25,381 | Adjusted prevalence ratio: 1.69 (95% CI, 1.02 to 2.79) (46) | 8 (4 to 13) | NNTH: 125 (77 to 250) | Observational evidence, potential for residual confounding, transient outcome |
| 376/15,729 (2.4%) versus 113/9652 (1.2%) (47) | AOR, 1.68 (95% CI, 1.34 to 2.12) (47) | ||||||||
| Women with depression | Persistent pulmonary hypertension of the newborn | Exposed to SSRIs during pregnancy | Unexposed to SSRIs during pregnancy | 94/54,281 (0.2%) versus 669/567,118 (0.1%) (48) | 621,399 | Adjusted OR, 1.28 (95% CI, 1.01 to 1.70) (48) | 1 (<1 to 1) | NNTH: 3031 (1220 to 11,112) | Observational evidence, potential for residual confounding |
| AOR, when not restricted to full‐term or by outcome (persistent pulmonary hypertension rather than primary persistent pulmonary hypertension): 1.08 (95% CI, 0.92 to 1.27) (48) | |||||||||
| Women with a mental health disorder | Childhood depression | Exposed to SSRIs during pregnancy | Unexposed to SSRIs during pregnancy | 60/15,729 (0.4%) versus 30/9651 (0.3%) | 25,380 | AHR, 1.78 (95% CI, 1.12 to 2.82) (49) | 2 (0 to 6) | NNTH: 414 (178 to 2703) | Observational evidence, potential for residual confounding |
| Women with a mental health disorder | Autism spectrum disorder without intellectual disabilities | Exposed to citalopram during pregnancy | Unexposed to any antidepressants during pregnancy | 46/1064 (4.3%) versus 291/12,325 (2.4%) | 13,389 | AOR, 1.75 (95% CI, 1.25 to 2.45) (50) | 17 (6 to 32) | NNTH: 59 (32 to 167) | Observational evidence, potential for residual confounding |
Comparative Effectiveness of Perinatal Pharmacotherapy
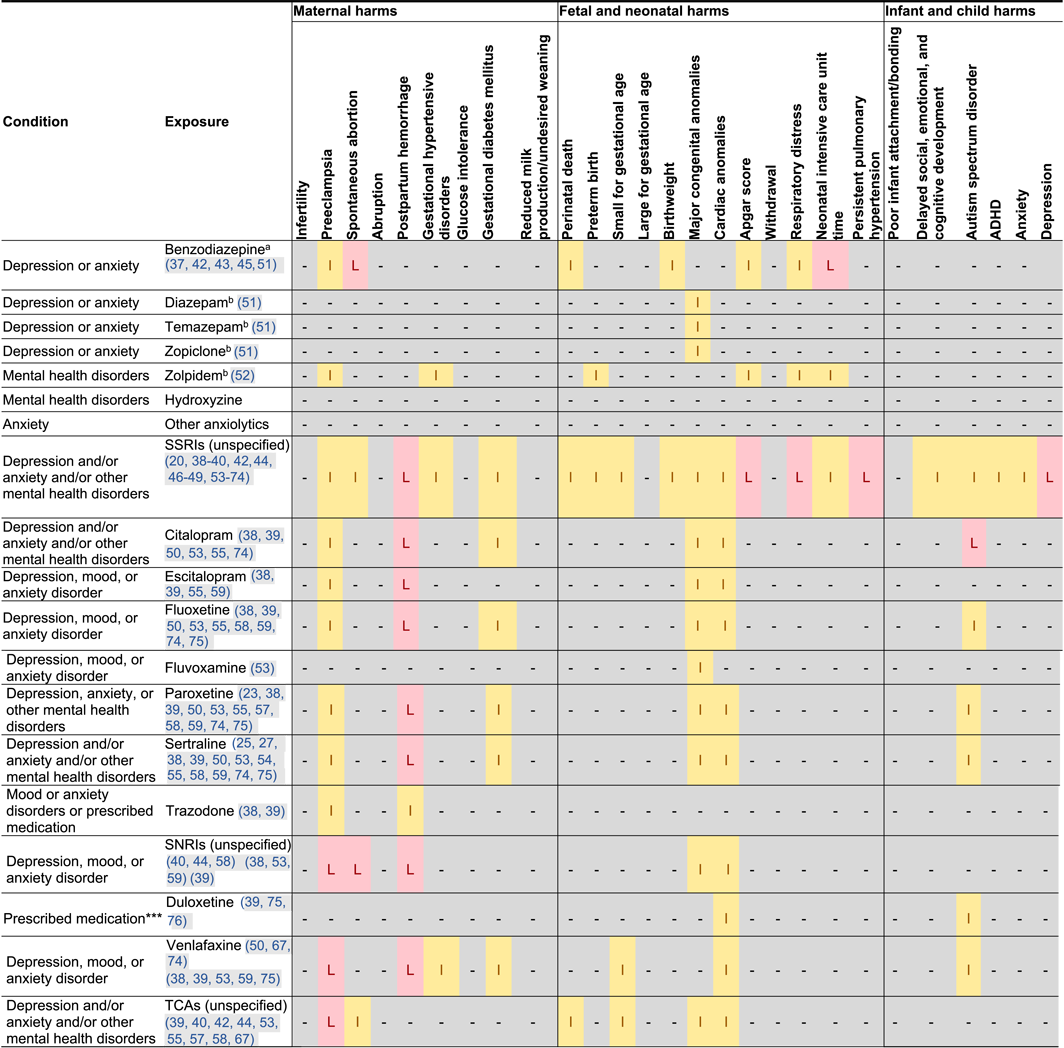
Harms of Perinatal Pharmacotherapy
 |
 |
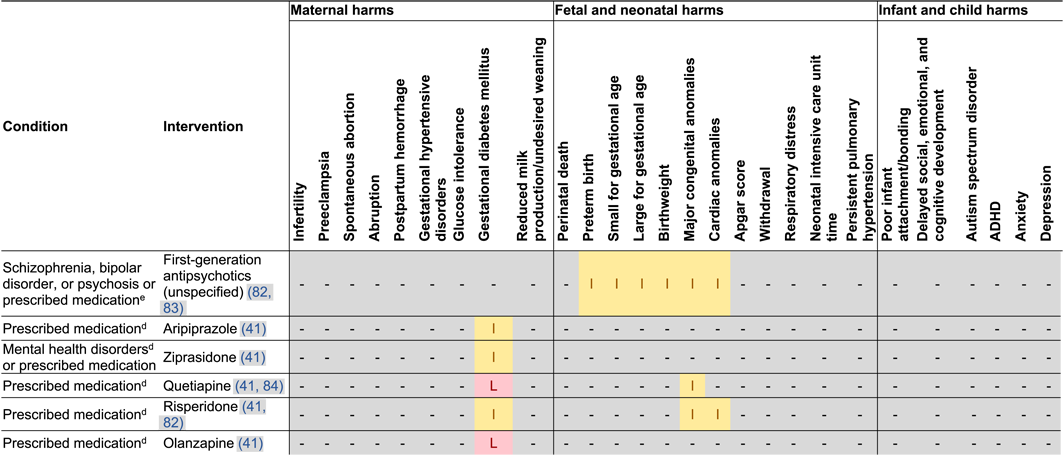 |
Maternal Harm
Postpartum Hemorrhage
Preeclampsia
Gestational Diabetes
Early Pregnancy Loss
Excessive Sedation or Loss of Consciousness
Fetal, Infant, or Child Harms
Persistent Pulmonary Hypertension of the Newborn
Congenital Anomalies
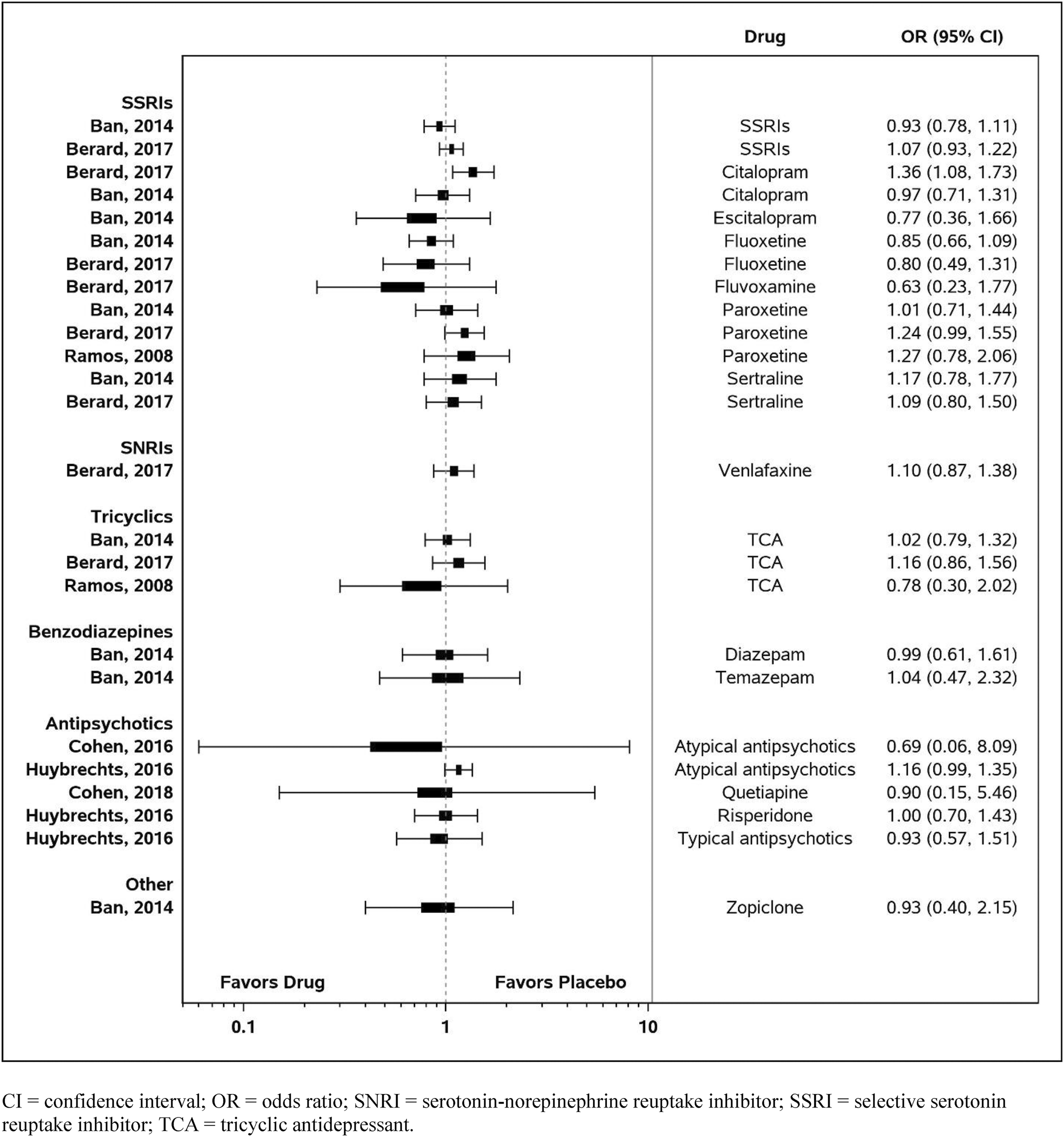
Comparative Harms of Perinatal Pharmacotherapy
DISCUSSION
Limitations
CONCLUSION
Author and Article Information
Supplementary Material
- Download
- 2.15 MB
REFERENCES
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
