Post‐traumatic stress disorder (PTSD) affects approximately 7% of people in the United States at some point during their life (
1,
2) and is much more common in specific subpopulations, such as veterans of war (
3). PTSD is twice as common in women compared with men (
1,
4) and is the result of experiencing a traumatic event or series of events, such as military combat, rape and sexual assault, automobile accidents, natural disasters, witnessing death or serious injury, or an unexpected death of a close friend or family member (
5,
6,
7,
8,
9). Symptoms of PTSD may include re‐experiencing the event through nightmares or flashbacks, avoiding reminders of the event, persistent arousal, such as having difficulties with sleep or always being on edge, and mood disturbances, including negative thoughts about oneself and feelings of social isolation (
1).
For some, symptoms of PTSD may last a few days or weeks, while for others it may persist as chronic PTSD and last years or decades following the traumatic event (
10).
Although PTSD is a prevalent and potentially debilitating condition, it affects only a portion of patients who experience trauma. Risk factors for developing PTSD include previous exposure to trauma, intensity of response to the traumatic event, lack of social support, trait anxiety, ethnicity, and socioeconomic indicators (
11,
12,
13).
Cognitive behavioral therapy is the most common form of post‐traumatic treatment designed to prevent the onset of PTSD and has been found to be only moderately effective (
14). In recent years, there has been an increased interest in the exploration of pharmacologic therapy for the prevention of PTSD (
14,
15). Following a traumatic event, the body experiences neuroendocrine changes, such as lower cortisol levels (
11), and it has been hypothesized that medications that impact the neuroendocrine system and regulation of stress‐related hormones could be promising candidates for future preventative treatments (
14). Additionally, potential prophylactic treatments may include pharmacotherapies that impact the formation of memories (
14). The most commonly proposed and studied medications include hydrocortisone (
16), propranolol (
17)
, benzodiazepines (
18) and morphine (
19); however, apart from moderate evidence for the benefit of hydrocortisone, prior research is limited and based on relatively small observational studies, pilot studies and/or animal models. Currently, the only FDA approved treatments for PTSD are two selective serotonin reuptake inhibitors (SSRIs), sertraline and paroxetine, which are effective in reducing symptoms of PTSD in some patients (
20) and have an overall positive safety profile with insomnia being the most prevalent adverse event (
21). The treatment guidelines for PTSD issued by the United States Department of Veterans Affairs (VA) and the Department of Defense (DoD) also include as recommended pharmacotherapies the SSRI fluoxetine as well as one serotonin‐norepinephrine reuptake inhibitor (SNRI), venlafaxine (
22). The VA and DoD additionally suggest the use of nefazodone (a serotonin antagonist and reuptake inhibitor), imipramine (tricyclic antidepressant) or phenelzine (monoamine oxidase inhibitor), if other therapies are ineffective. Like many other diseases, a “one‐size‐fits‐all” approach where the only approved therapies are from a single treatment class is not ideal. This is especially true for a condition as diverse as PTSD where patients can vary substantially on age, gender, comorbidities, and type of trauma experienced. There is a gap in knowledge about what treatments are most effective for which patients and in what ways they are effective (e.g., quality of life is largely unstudied). Despite advances in the understanding of the pathophysiology of PTSD and evidence suggesting alterations in neural circuitry, molecular biology, endocrinology and immune reactivity, there are no new drug targets, and the only medications that are FDA approved or included in treatment guidelines are those agents that have been developed for other indications. There is a need to develop more effective and targeted treatments for PTSD based on this increasing biological understanding (
23).
Explorations into new effective PTSD treatments have typically relied on a theory driven approach, which first hypothesizes how PTSD biologically manifests in trauma survivors and then identifies existing medications that may mediate that pathway (
14). However, there is potential to look at a much broader landscape of medications. By leveraging retrospective observational data, it is possible to examine the association between all existing medications and the incidence of PTSD to uncover potential targets for new drug development.
Retrospective observational data, such as that from administrative health insurance claims, has historically been used to inform regulatory decision‐making regarding the safety and effectiveness of existing drugs (
24,
25). We created the Real‐World Assessment and Research of Drug Performance (REWARD) framework, which utilizes hundreds of millions of patient records to study the association between all medications with thousands of outcomes. This framework has recently been used to identify unknown benefits of existing drugs and thereby help to inform new drug development in neuroscience (
26,
27,
28). In this study, the association of all existing medications with the incidence of diagnosed PTSD was examined by leveraging administrative claims data from four large US‐based claims databases. Results of this study may be used to generate novel hypotheses aimed at the development of more effective medications for the treatment and/or prevention of PTSD.
Methods
A self‐controlled cohort design in which individuals serve as their own controls was used. This design tends to produce less biased estimates with higher predictive accuracy than other more commonly used designs, such as case‐control studies (
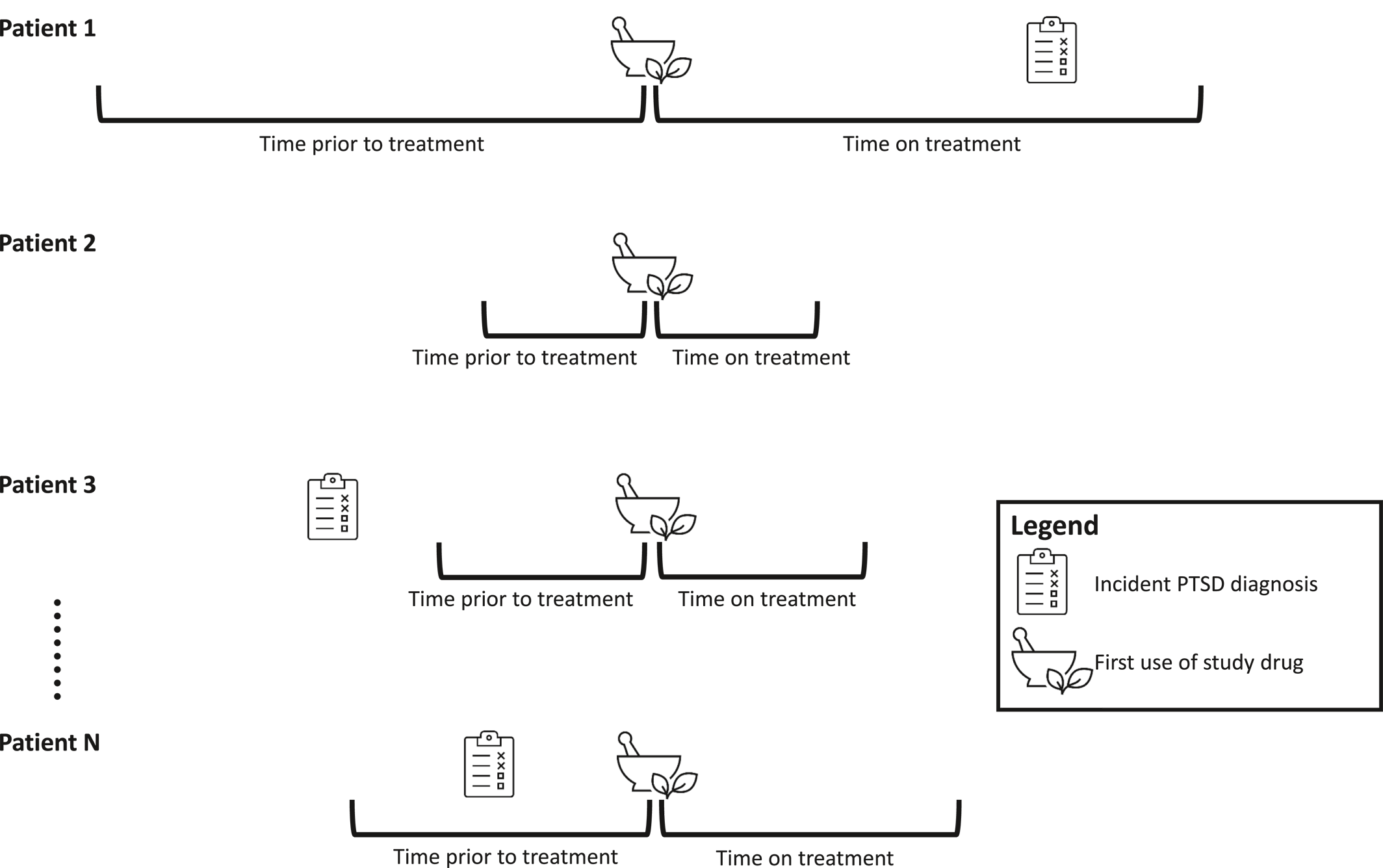
29). An illustration of the study design for a single medication can be found in
Figure 1. A separate analysis was conducted for every medication in each of the four databases. That is, there were 1399 analyses (one for each medication) conducted in each of the four databases—a total of 5596 analyses. For each analysis of a specific medication, all patients receiving that medication were included. Patients receiving multiple medications over time were included in multiple analyses, with their index date corresponding to the initiation date of the drug being studied. The REWARD framework has been used in previous studies examining treatments associated with the risk of parkinsonism (
27), dementia (
28), and depression and bipolar disorders (
26).
Data Sources
The analysis was executed in four US‐based administrative claims databases. Each database contains data from adjudicated health insurance claims (e.g., inpatient, outpatient/emergency department, and outpatient pharmacy) and health plan enrollment information. Briefly, the four databases included in this study were:
1.
IBM® MarketScan® Commercial Database (CCAE): Includes data from 154 million individuals enrolled in employer‐sponsored insurance health plans, during January 1, 2000, through April 30, 2020.
2.
IBM® MarketScan® Multi‐State Medicaid Database (MDCD): A claims database for 29 million Medicaid enrollees from multiple states during January 1, 2006, through December 31, 2019. Examples of patients that are eligible for Medicaid coverage include low‐income families, qualified pregnant women and children, and individuals receiving Supplemental Security Income (
30).
3.
IBM® MarketScan® Medicare Supplemental Database (MDCR): Includes data for more than 10 million retirees with primary or Medicare supplemental coverage through privately insured fee‐for‐service, point‐of‐service, or capitated health plans during January 1, 2000, through January 31, 2020.
4.
Optum© Clinformatics® Data Mart. Includes 86 million members with private health insurance, who are fully insured in commercial plans or in administrative services only and Medicare Advantage (Medicare Advantage Prescription Drug coverage. The population is representative of US commercial claims patients (0–65 years old) with some Medicare (65+ years old) during May 1, 2000, through Mar 31, 2020.
Data elements included were outpatient pharmacy dispensing claims (coded with National Drug Codes) as well as inpatient and outpatient medical claims, which provide diagnosis codes (coded in ICD‐9‐CM or ICD‐10‐CM). The use of the IBM MarketScan and Optum claims databases was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subjects research.
Exposure and Control Definition
Medications were identified according to the RxNorm ingredient. All RxNorm ingredients received by patients in any of the databases during the study period were included (n = 1399 unique ingredients) and analyzed independently. Individuals were identified at the time they first filled the medication of interest. An exposure period was defined as the period starting with initiation of the medication until discontinuation or end of observation, allowing for a gap of the medication supply plus 30 days between consecutive fills. The time directly preceding the exposure and equal in length to the exposure period served as the unexposed (i.e., control) period.
Medications commonly used for treating PTSD or its symptoms, such as antipsychotics, antidepressants and mood stabilizers, were excluded from the analysis due to the confounded relationship to the outcome introduced by the study design. Because these medications are used to treat the condition, exposure to these medications will typically come after the first diagnosis of PTSD and rarely prior. This leads to a much higher incidence of the condition in the unexposed period compared with the exposed period and thus artificially reduces the relative risk when using this study design.
Outcome Definition
Subjects with an incident PTSD diagnosis were identified as those having at least two claims containing a diagnosis for PTSD (ICD‐9‐CM code 309.81 or ICD‐10‐CM codes F43.10, F43.11, F43.12) on distinct service dates and occurring within 12 months of each other. The date of the first claim was considered the outcome date. A study examining the validity of identifying PTSD patients in the VA National Patient Care Database using two claims with an ICD‐9‐CM code for PTSD (309.81) yielded a positive predictive value of 81.8% when compared with the self‐reported PTSD Checklist (PCL) (
31).
Statistical Analysis
For each treatment, incidence rates (IR) of PTSD were calculated for the exposed and unexposed periods, and an incident rate ratio (IRR) was calculated as the IR in exposed divided by the IR in unexposed. An IRR >1.0 indicates more cases identified after initiation of the medication, while an IRR <1.0 indicates fewer cases identified after initiation.
The IRRs, corresponding 95% confidence intervals, and p‐values were calibrated using negative controls to adjust for residual bias. Negative controls included medications that have no evidence of association with PTSD according to product labels, spontaneous event reports or PubMed indexed publications using the Common Evidence Model, which builds upon the OHDSI Knowledgebase (
32). A self‐controlled cohort study was performed for each of the negative controls, and the effect estimates were captured. Characteristics of this empirical distribution (i.e., mean and variance of the effect estimates) were used to calibrate all associations examined in this study. For example, if the distribution of negative controls showed a mean estimate <1.0, then it was assumed that all associations were biased towards a protective association, and calibration was used to adjust these associations in the opposite (non‐protective) direction.
Following calibration of the effect estimates, confidence intervals and p‐values, strict filtering criteria were applied to identify medications with potential protective benefits in PTSD:
1.
The medication must have been associated with a reduction of ≥30% (moderate effect) or ≥50% (strong effect), with p < 0.05, for the incidence of PTSD, in at least two databases; and
2.
There must have been no evidence of increased risk between the medication and PTSD in any of the databases, defined as having an IRR >1.3 with p < 0.05.
The calibrated results were reported for analyses in each of the four databases. A meta‐analysis using a mixed‐effects Poisson regression model with random study effects was then performed to pool results across the four databases into a single effect estimate and 95% confidence interval. The I2 measure was used to measure heterogeneity of the associations across the data sources.
Results
A total of 137,182,179 individuals were included in the analysis across the 4 databases. The average age of patients diagnosed with PTSD varied across the databases, with the youngest being represented in the MDCD database (mean age 28.2 years) and the oldest being represented in the MDCR database (69.9 years). Approximately two‐thirds of patients diagnosed with PTSD were women in the CCAE, MDCD and Optum databases, while in the MDCR database, most PTSD patients (58.2%) were men.
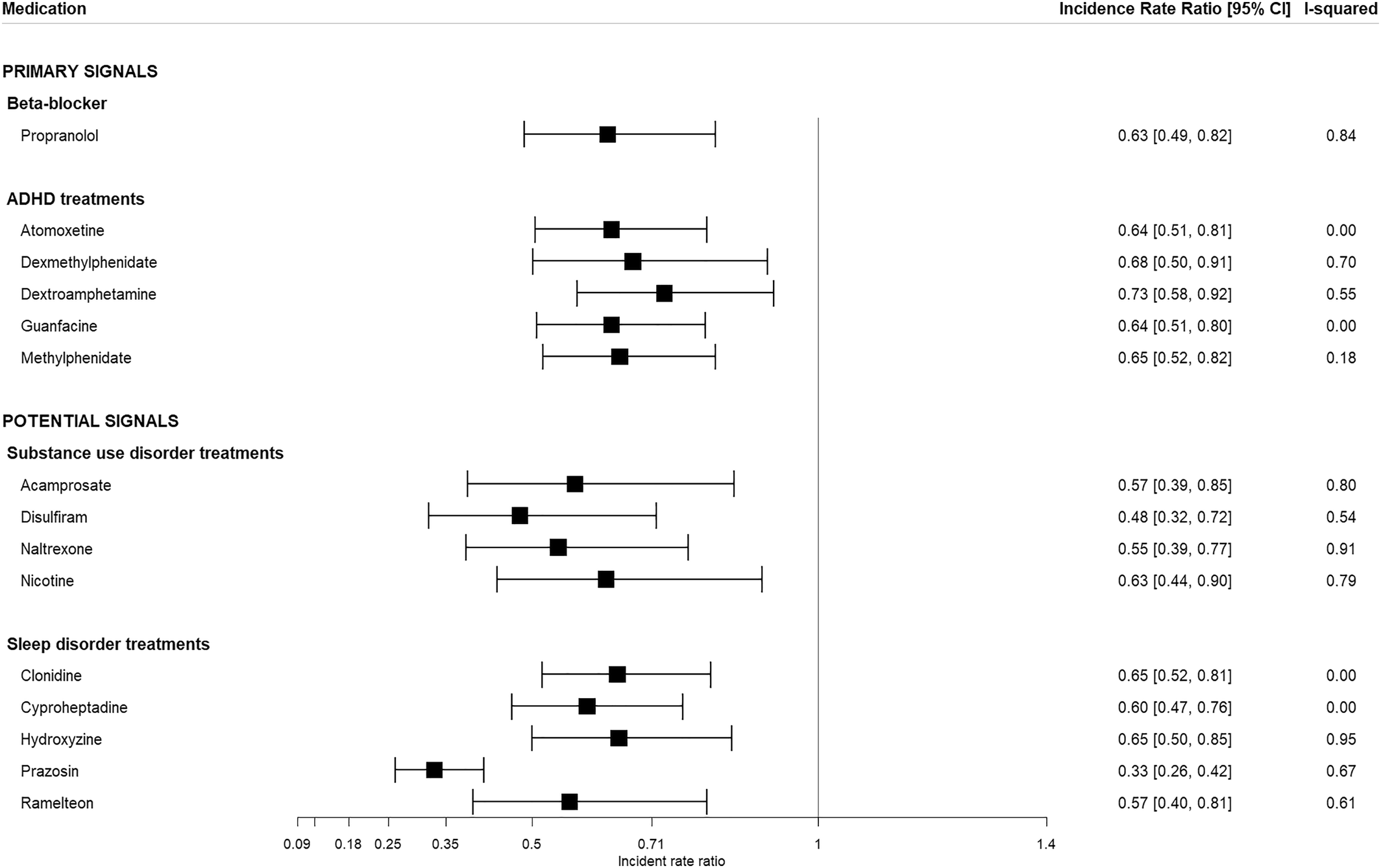
There were 1399 individual medications studied, 15 of which met the threshold criteria described above (
Table 1). Of the 15 medications identified, six are categorized as “primary signals” while the remaining nine are considered “potential signals”. The primary signals include medications that have been previously investigated or proposed as potential therapies for PTSD but are not commonly used for this purpose. The potential signals include medications that showed strong protective effects. However, this may be due to off‐label use or the treatment of PTSD symptoms.
The six primary signals and the associated calibrated meta‐analysis IRRs [95% CI] include the beta blocker propranolol (0.63 [0.49–0.82]) and five medications used to treat attention‐deficit/hyperactivity disorder (ADHD— atomoxetine (0.59 [0.55–0.63]), dexmethylphenidate (0.62 [0.51–0.76]), dextroamphetamine (0.67 [0.63–0.72]), methylphenidate (0.60 [0.57–0.63]) and guanfacine (0.59 [0.56–0.62]) (
Figure 2). The nine potential signals include drugs that are indicated for the treatment of sleep disorders/sleep disturbances and may have been used off‐label to treat PTSD (clonidine, cyproheptadine, hydroxyzine, prazosin and ramelteon) as well as drugs used to treat substance use disorders (acamprosate, disulfiram, naltrexone, and nicotine), which are common among patients diagnosed with PTSD. These are considered potential signals because the effect estimates may be biased by the study design (i.e., their occurrence almost always follows a diagnosis of PTSD, since they are used as treatments for the symptoms of the underlying disease, leading to artificially low IRRs).
A full list of calibrated and uncalibrated results within each of the databases and the pooled effects estimates from the meta‐analysis can be found in Table
S1.
Discussion
This study examined the association between more than 1000 medications and incident PTSD across four US administrative claims databases, identifying 15 medications that showed strong, consistent, protective associations with PTSD, six of which have previously been investigated or proposed as potential treatments. The pharmacological signals generated by the approach taken here provide an important first step to potentially discovering new, effective medications to treat and/or prevent PTSD.
One drug identified in this study, propranolol, was examined previously as a potential treatment for PTSD nearly 2 decades ago (
17,
33) and, more recently, via meta‐analysis (
34) and a clinical trial (
35). Propranolol has been hypothesized to be a potential deterrent of the development of PTSD by virtue of its inhibitory effects on memory reconsolidation; however, the totality of current evidence regarding its effectiveness is mixed (
36). It is possible that some of the effect seen in this study could be due to its off‐label use to treat PTSD, but this is likely very rare compared to the overall rate at which propranolol is prescribed for other conditions. The remaining medications evincing the strongest effects sizes in this study, atomoxetine, dexmethylphenidate, dextroamphetamine, methylphenidate, and guanfacine, are all indicated for the treatment of ADHD and include stimulant as well as non‐stimulant drugs. Each of these medications has been proposed as a potential treatment for PTSD patients (
37,
38,
39,
40), though evidence from human trials is relatively scarce. These medications are thought to potentially inhibit PTSD through various mechanisms, including the enhancement of brain dopaminergic activity, the reduction of inflammatory cytokines and the strengthening of prefrontal cortical norepinephrine connectivity (
37,
39,
40). Individuals with ADHD have a nearly 3‐fold increased risk of developing PTSD (
41), warranting further research within this population. Such a study may include an examination of whether ADHD patients who are treated with these (and similar) medications have lower rates of incident PTSD compared with ADHD patients who are treated with other therapeutic drug classes. While these medications have been identified here as the primary signals, there are ways in which bias and confounding due to the self‐controlled study design could be contributing to the observed protective effect estimates. Individuals with ADHD may be at an increased risk of trauma due to risk‐taking behaviors (
42). If these behavior occur more often in those who are untreated it may lead to PTSD prior to them receiving treatment for their ADHD. If this scenario plays out over many patients it can result in a biased effect estimate in the protective direction. This illustrates a limitation of the self‐controlled cohort design in general–medications used to treat conditions related to PTSD (whether comorbidities, risk factors, or consequences of the PTSD) which are commonly given
after the PTSD is diagnosed may produce estimates biased in the protective direction. Conversely, if these medications are commonly given prior to PTSD being diagnosed, the estimates will be biased towards an increased risk.
Four of the signals in this study comprised medications that are used to treat addictive disorders, highlighting a potential shared pathway between the etiology of PTSD and the development of addictive behavior. Substance use disorders are commonly observed in patients diagnosed with PTSD, co‐occurring in anywhere from 25% to 73% of individuals with PTSD (
43). If there exists a shared biologic pathway between the development of PTSD and substance use disorders, then it is logical to hypothesize that treatments for one condition may be effective for treating the other. However, it is important to note that substance use disorders often manifest after the onset of PTSD, potentially through an attempt to “self‐medicate” (
44), and the protective effects found in this study may be driven in part by a confound of the study design (i.e., because PTSD often precedes the onset of a substance use disorder and, therefore, the treatment for the substance use disorder, the relative effect estimate will be artificially lower than the true effect of the medication on the outcome of PTSD). To overcome this limitation, future research on the association between these medications and the onset of PTSD may wish to examine a subset of patients diagnosed with substance use disorders and compare rates of subsequent PTSD in those that receive these treatments versus those who do not.
While the methods used in this study are appropriate for causal inference, the present research was designed to identify a manageable number of hypotheses of biological pathways, which can then be studied further, not to confirm a causal association. The pharmacotherapies identified here may benefit from additional observational research implementing study designs tailored to the specific exposure of interest. A major strength of the present study is the use of a self‐controlled design, which allowed individuals to serve as their own control and thus control for any time‐invariant covariates, such as genetics. Moreover, the analysis included 5596 statistical models, each with a unique combination of exposure, outcome and database; and strict filtering criteria were applied to avoid erroneous findings due to multiple comparisons and narrow confidence intervals. For example, it was required that the medication must have been associated with at least a 30% reduction in the incidence of PTSD, a large effect in observational research, in at least two of the four databases.
The self‐controlled cohort design does not control for time‐varying influences, including characteristics such as comorbid conditions, medications and procedures received, changes in general health status and age. Many of these factors are typically associated with a worsening of health as time passes; for example, as a function of age, more comorbidities are diagnosed, and more medications are prescribed. In the self‐controlled design these factors would typically bias results towards showing a risk (i.e., negative outcomes associated with worsening health would occur more often after starting a drug purely due to a longer time duration), and typically, it would not be expected that these variables would bias results towards a protective effect of the medication. However, there are situations where the self‐controlled cohort design may bias results towards a protective effect. For instance, drugs that are used to treat PTSD or conditions caused by PTSD, and therefore create an exposure after a diagnosis of PTSD, will typically show up as protective solely because PTSD is being diagnosed prior to these medications being prescribed, not necessarily because the medications are preventing the incidence of a future PTSD diagnoses.
Another limitation of this study is that the evidence gathered and analyzed was based on imperfect administrative claims data, though the diagnosis codes used to identify PTSD have produced good measures of validity, according to positive predictive values, especially when requiring a second, confirmatory diagnosis, as was done in this study (
31). While this validation provides confidence that when diagnosis codes are present for PTSD it is highly likely the patient truly has PTSD, it does not account for the potentially low sensitivity of PTSD being diagnosed in a clinical setting (
45). Therefore, the patients identified in this study as having PTSD may reflect a more severe group of PTSD patients compared with the overall PTSD population.
In addition, the self‐controlled cohort analysis may not be ideal for chronic outcomes, due to the limited amount of time patients remain on a medication after initiating therapy. However, patients with exceptionally short exposure periods added little information to the analysis, due to observation periods being too short to capture outcomes; and thus, the individuals who contributed the most information were those with long exposure periods.
Conclusion
There is a large unmet need for medications that are effective at preventing or treating PTSD. The few currently approved treatments are antidepressants that are limited in their efficacy for treating the totality of symptoms associated with PTSD and do not prevent incidence of the condition. This study leveraged a vast amount of observational data to perform large‐scale analytics across multiple databases. The associations between nearly 1400 drugs and the outcome of incident PTSD were assessed, and a handful of signals were detected, which may be candidates for further investigation. This approach provides tangible targets for more rigorous research that can aid in the discovery of new and effective treatments not only for PTSD but also other diseases for which the unmet medical need remains high.