The Longitudinal Assessment of Manic Symptoms (LAMS), funded by the National Institute of Mental Health, was designed to follow prospectively an epidemiologically ascertained cohort of children with elevated symptoms of mania receiving outpatient care, as well as a comparison group of children without such symptoms also receiving outpatient care. The study was designed both to delineate the relationship between manic symptoms and bipolar disorder and to carefully define the characteristics of children with elevated symptoms of mania. Children with mood and behavior disorders, such as those experienced by children in the LAMS sample, are frequently treated with one or more psychotropic medications (
1), but there is also evidence that many youths with diagnosable psychiatric disorders receive no treatment (
2).
The pediatric use of psychotropic medications has been criticized in the media (
3) and scientific literature (
4,
5). Critics have cited a lack of substantive evidence as to their efficacy, effectiveness, and safety for children and adolescents. However, most previous studies have analyzed claims data and have not included thorough assessments of diagnosis, service utilization, comorbid disorders, and other important clinical factors, such as functioning. Constantine and colleagues (
6) analyzed data from a Florida Medicaid database and found that 7% of children and 8% of adolescents had at least one episode of receiving two or more psychiatric medications during the five-year period (July 2002 to June 2007). They reported that the following factors increased the odds of receiving two or more psychiatric medications: being an adolescent (odds ratio [OR]=1.16), race indicated as “other” (OR=1.18), and presence of psychosis (OR=1.47). Zito and colleagues (
4) reviewed Texas Medicaid data for a July 2004 random sample of 472 medicated youths in foster care age 0 through 19 years. In this sample, the average number of psychiatric medications received by each child was 2.55, and 41% received three or more classes of psychotropic medications.
LAMS used an epidemiological approach to assemble a cohort of children age six to 12 years at their first outpatient mental health visit at university-affiliated clinics (
7). Initial results have confirmed the finding that elevated symptoms of mania in mental health settings are in fact common and that most children with such symptoms meet
DSM-IV criteria for a variety of disorders other than a bipolar spectrum disorder at baseline (
1).
The LAMS sample was thoroughly assessed at baseline with state-of-the-art research instruments for primary psychiatric disorders, comorbid disorders, level of functioning, and medication use. It is important to understand that LAMS is a longitudinal observational study and not a treatment study. LAMS participants were screened and recruited during their first mental health visit at clinics associated with four academic medical centers in the Midwest. Most LAMS participants were treated in their local community, and in most cases the LAMS team clinicians provided no feedback about diagnosis or treatment provided to the child’s prescribing clinician. Thus these results reflect community practices rather than a controlled trial. This combination of a naturalistic epidemiological design with research-grade description of diagnoses and service utilization offers a crucial window into the debate about current clinical patterns of prescription.
The purpose of this analysis was to use the LAMS cohort to examine factors associated with polypharmacy in the outpatient treatment of youths referred because of emotional and behavioral concerns. We examined bivariate relationships between demographic and clinical characteristics and the number of medications the youth was taking concurrently. Hierarchical logistic regression analyses quantified the extent to which sets of demographic and clinical characteristics contributed to the odds of polypharmacy. We also tested whether any factors demonstrated unique incremental effects. We hypothesized that diagnosis and severity would significantly increase the odds of polypharmacy. Because polypharmacy carries increased risk of side effects, adverse events, and fiscal costs, we approached the analyses as an investigation of potential harm. Thus we did not use a post hoc correction, which would have reduced power to detect potentially important predictors of polypharmacy. For example, if a variable was associated with polypharmacy with an uncorrected p value of .04, post hoc correction would reclassify it as nonsignificant, whereas not using a post hoc correction would flag the variable for discussion.
Methods
Study sites and participant ascertainment
All study procedures were reviewed and approved by the institutional review boards at each of the four university-affiliated sites where LAMS is being conducted. Written informed consent from parents or guardians and assent from youths were obtained before any study-related procedures were performed.
The LAMS source population consisted of all children between the ages of six and12 years and 11 months who were making a first visit to nine child outpatient clinics associated with the four university-affiliated medical centers in the LAMS study. Parents or guardians accompanying eligible children were approached by researchers using procedures approved by the institutional review board at each university or hospital. Recruitment occurred between December 13, 2005, and December 18, 2008. After providing consent to participate, adults were asked to complete the ten-item Parent-Completed General Behavior Inventory Mania Form (P-GBI-10M) (
8) and to answer questions about sociodemographic characteristics.
Of 3,329 families who were approached at the participating clinics, 2,622 (79%) agreed to screening. Nearly half (N=1,124, 43%) scored above the a priori cutoff of 12 on the P-GBI-10M, indicating that the child had elevated symptoms of mania. The threshold of 12 was deliberately set low, both to increase sensitivity to bipolar spectrum disorders and to include a wide variety of cases not meeting criteria for bipolar disorder in order to investigate the course of cases that might have some features similar to those of mood disorders. Of the 1,124 children, 1,111 were eligible for the longitudinal follow-up portion of the study; 13 were ineligible because the parent reported a diagnosis of autism or an IQ of less than 70. Of the 1,111 eligible children with elevated symptoms of mania, 621 parent-child dyads (56%) agreed to participate.
For every ten children with elevated symptoms of mania, one child without such symptoms (score below 12 on the P-GBI-10M) was selected as a potential comparator. A total of 86 parent-child dyads in which the child was negative for elevated symptoms of mania agreed to participate, resulting in an overall sample of 707 children and their parents. Information on the study design, sample selection, and children’s sociodemographic characteristics has been previously reported (
7). Rates of attention-deficit hyperactivity disorder (ADHD), anxiety disorders, psychosis, elimination disorders, and depression were similar between the 86 children without elevated symptoms of mania and the 621 children who met criteria for such symptoms at screening, but the former group had significantly lower rates of bipolar disorders (N=7, 8%) and disruptive behavior disorders (N=30, 35%). Families who agreed to participate in the longitudinal portion of the study were scheduled for a baseline interview, which has been described in detail elsewhere (
1).
Measures
Demographic characteristics.
Parents or guardians provided demographic information, including child age, sex, race, ethnicity, and health insurance status, as well as information on family composition, socioeconomic status, parental education and employment, and medical history.
Psychiatric diagnoses.
To assess for current and past psychiatric disorders, children and their parent or guardian were administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (
9) (K-SADS-PL), with additional items about depression and manic symptoms derived from the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (
10). Items screening for
DSM-IV criteria for pervasive developmental disorders were also added to the K-SADS-PL. The resulting instrument, the K-SADS-PL-W, is a semistructured interview that assesses current and lifetime psychiatric diagnoses and the time course of each illness. The presence or absence of mood disorder diagnoses was assessed at the baseline evaluation and every six months thereafter. The entire K-SADS-PL-W was repeated annually.
Unmodified
DSM-IV diagnostic criteria were used in the LAMS study. The criteria for bipolar disorder not otherwise specified were clarified for the LAMS study to follow the same criteria used in the Course and Outcome of Bipolar Youth study (
11). Bipolar disorder not otherwise specified was operationalized as follows: elated mood plus two associated symptoms of mania (for example, grandiosity, decreased need for sleep, pressured speech, racing thoughts, and increased goal-directed activity) or irritable mood plus three associated symptoms of mania; change in level of functioning; presence of symptoms for a total of at least four hours within a 24-hour period; and at least four episodes of four hours’ duration or a total of four days of the above-noted symptom intensity in the child’s lifetime. All diagnoses were reviewed and confirmed by a licensed child psychiatrist or psychologist.
For the analyses reported here, eight summary variables indicated whether the youth had a diagnosis of ADHD, bipolar spectrum disorders, depression or dysthymia, anxiety disorders, disruptive behavior disorder (oppositional defiant disorder or conduct disorder), elimination disorders, pervasive developmental disorder, or a history of psychosis (including psychosis during mood episodes as well as schizophrenia). We treated psychosis as a separate variable because it might separately predict polypharmacy above and beyond the main effects of bipolar or depressive disorders.
Functional assessment.
Study interviewers completed the Children’s Global Assessment Scale (CGAS) to provide a severity rating of participants’ current impairment, measuring overall functional capacity at home, at school, and with peers (
12).
Symptomatic assessment.
In addition to administration of the K-SADS-PL-W, which ascertained presence or absence of manic and depressive symptoms specifically within the context of a mood episode (that is, “filtered” ratings), “unfiltered” ratings of apparent mood symptoms regardless of whether they occurred within the context of a mood episode were also assessed via both parental self-report and clinical rating. Manic and behavioral dysregulation symptoms, regardless of etiology, were assessed by parent report with the P-GBI-10M as well as with the Young Mania Rating Scale (YMRS) (
13), which was administered to both the parent and child. The P-GBI-10M and YMRS scores were correlated (r=.43, p<.001). Similarly, the presence and severity of depressive symptoms, regardless of etiology, were assessed with the Children’s Depression Rating Scale–Revised (CDRS-R) (
14). Parent-reported scores on the ADHD, oppositional defiant disorder, and conduct disorder subscales were examined with the Child and Adolescent Symptom Inventory 4R–Parent Version (
15). Finally, the total score on the four-item outward irritability subscale of the Irritability, Depression, Anxiety Scale (
16) was used to measure irritability.
Family factors.
The Family History Screen (
17) was administered at the baseline assessment to collect information on 15 psychiatric disorders and suicidal behavior of biological parents and of first- and second-degree relatives. The analyses reported here concentrated on two summary variables capturing whether the mother or father had a history of a mood disorder or cognate diagnoses potentially associated with a familial history of mood disorder, such as schizophrenia, antisocial personality disorder, or substance abuse or dependence (
18). Thirty-two percent (N=224) of children lived with both biological parents.
Mental health services use.
The parent version of the Service Assessment for Children and Adolescents (SACA) (
19,
20) was used to gather information about the child’s lifetime and current use of various mental health services in three broad domains: inpatient, outpatient, and school. Parents or guardians were asked to rate how well the most recent outpatient services matched their child’s needs and how much their child had benefited from the most recent treatment.
Medication history.
As part of the SACA, each child’s parent or guardian provided a complete history of the child’s past and currently prescribed psychotropic medications. Medications were grouped according to class (mood stabilizers, antidepressants, antipsychotics, stimulants, and “other”). For simplicity, lithium and anticonvulsants were classified as mood stabilizers. Current medication use was quantified in several ways: the number of medications taken simultaneously, a dummy code indicating whether the person was receiving two or more medications concurrently, and dummy codes for inclusion in regression models indicating whether the youth was taking an antipsychotic, a stimulant, an antidepressant, or a mood stabilizer.
Statistical analyses
Data were double-entered using SPSS Data Builder/Entry, version 3. Statistical analyses were conducted with SAS, version 9.2, and SPSS, version 19. Descriptive statistics included examination of skewness and kurtosis. Chi square analyses tested associations between levels of medication use and categorical variables, and one-way analyses of variance tested bivariate associations with continuous measures. This framework allowed for potential nonlinear effects for medication augmentation.
Polypharmacy was operationally defined as taking two or more psychiatric medications concurrently. It served as the dependent variable in logistic regression models testing the predictors in hierarchically organized blocks. As a sensitivity analysis, the number of concomitant medications was the dependent variable in a set of Poisson regressions to evaluate whether the predictors of number of medications were similar to the predictors of polypharmacy. All hypothesis tests were considered statistically significant if the p value was <.05. We elected not to use a more conservative alpha level because we were specifically interested in predictors of higher medication usage, and thus more stringent alpha levels might prevent detection of important predictors of polypharmacy (
21). Post hoc error correction would make it more difficult to detect predictors of polypharmacy. The study was designed to provide adequate statistical power for longitudinal follow-up of cases to estimate rates of diagnostic change. Based on the obtained sample size, with the alpha set at .05, the study had 80% power to detect very small effect sizes (Cohen’s d values of ≥.11 for t tests and Cohen’s w values of ≥.065 for chi square tests).
Results
Preliminary analyses and sample characteristics
Complete information on the variables of interest for these analyses was available for 698 of the 707 participants. Little’s test indicated that the missing-completely-at-random assumption was tenable. Because the assumption was tenable and the amount of missing data was small (<2% on all relevant variables), we used listwise deletion. Therefore, the number for all analyses was 698 unless otherwise indicated.
The LAMS sample has been previously described (
1,
7). Briefly, approximately two-thirds of the 707 children were male (N=478, 68%) and white (N=455, 64%). Nearly half (N=323, 46%) were between the ages of six and eight. A total of 370 children (52%) were insured solely through Medicaid. Primary diagnoses in the sample were bipolar disorders (N=162, 23%), depressive disorders (N=124, 18%), psychotic disorders (N=10, 1%), anxiety disorders (N=43, 6%), disruptive behavioral disorders (N=212, 30%), ADHD (N=91, 13%), and pervasive developmental disorders (N=28, 4%). A total of 433 children (61%) had mild functional impairment, and 435 (62%) had taken psychotropic medications during their lifetime. Eighteen youths were presenting for evaluation only and were not taking any medication. As mentioned above, the youths without elevated symptoms of mania were less likely than those with such symptoms to have a diagnosis of a bipolar disorder or a disruptive behavior disorder; a larger proportion of the youths without elevated symptoms had insurance other than Medicaid, but this difference was not significant. Otherwise, no significant diagnostic or demographic differences were noted between the groups with and without elevated symptoms of mania.
Table 1 lists the demographic and clinical characteristics of the study sample and presents data on bivariate associations by medication group (no medications and one, two, or three or more medications).
A total of 201 of the 698 participants (29%) were prescribed two or more psychotropic medications. LAMS participants receiving two or more medications had lower CGAS scores, more comorbid disorders, and higher baseline P-GBI-10M, YMRS, and CDRS-R scores than LAMS participants treated with no or one medication; the former group was also were more likely to have a bipolar or pervasive developmental disorder, to have been placed in special education, and to have been psychiatrically hospitalized, suggesting that children who received two or more medications represented complex and difficult-to-treat cases. The proportion of males in the group taking three or more medications was significantly larger than in the group taking no medications. In addition, the proportion of white youths in the group taking two medications or three or more medications was significantly larger than in the group taking no medications (all p<.05 based on post hoc comparisons). A larger proportion of participants in the group taking no medication had Medicaid insurance, but the difference was statistically significant only for the comparison with the group taking two medications. Otherwise, no differences in demographic or insurance variables were found between the children receiving two or more medications and those receiving no or one medication.
Of the 262 participants who had not been prescribed any medications, 252 (96%) had one or more diagnoses of a psychiatric disorder. The most common disorders reported for the 262 children included ADHD (67%), disruptive behavior disorders (56%), anxiety disorders (36%), depressive disorder (21%), and elimination disorders (20%). Of the 262 children, 74% (N=194) had two or more psychiatric diagnoses. Children insured by Medicaid were less likely to receive multiple medications for a psychiatric disorder than children not insured by Medicaid. Post hoc sensitivity analyses found that 18 children (3% of the sample) were seeking only evaluation and not treatment and were not taking medication. Excluding these cases and rerunning the analyses did not change any of the estimates to two decimal places.
Post hoc analyses found that participants treated with two or more medications were more likely to be treated by a psychiatrist than by a pediatrician or a family practitioner (χ
2=5.37, df=1, p=.02). [A table listing the types of prescribing clinician for the groups taking one, two, or three or more medications is available as on online
data supplement to this article.]
Correlates of higher rates of polypharmacy
The finding that white youths were more rather than less likely to be prescribed two or more medications was not consistent with some previous findings (
6,
22). Therefore, we conducted a set of exploratory analyses to better characterize the robustness and nature of this finding. First, we examined outlier diagnostics to determine whether any cases exerted undue effects on the regression models. This was not the case. Second, we looked at bivariate associations between white race and other variables to examine whether they followed expected patterns. They did: with moderate unadjusted associations with site, point-biserial correlations between white race and other variables ranged from –.40 (white youths less likely to be insured by Medicaid,) to .25, for the total number of medications prescribed; .18, for use of antipsychotics; .15, for antidepressants; .13, for anxiety diagnoses (that is, a larger proportion of white participants with an anxiety diagnosis); .12, for mood stabilizers; .12, for seeing a psychiatrist; .10, for pervasive developmental disorders; –.08, for disruptive behavior disorders; –.08, for ADHD; .08, for seeing a psychologist; and .08, for overall number of diagnoses (p<.05 for all r
pb values; p<.005 for r
pb greater than .11 or less than –.11).
Finally, we conducted a set of logistic regressions exploring patterns of usage for the four medication classes, evaluating which sets of predictors accounted for the most variance in usage and also testing whether controlling for other demographic, clinical, and provider characteristics reduced the higher odds of polypharmacy among white youths.
Tables 2,
3, and
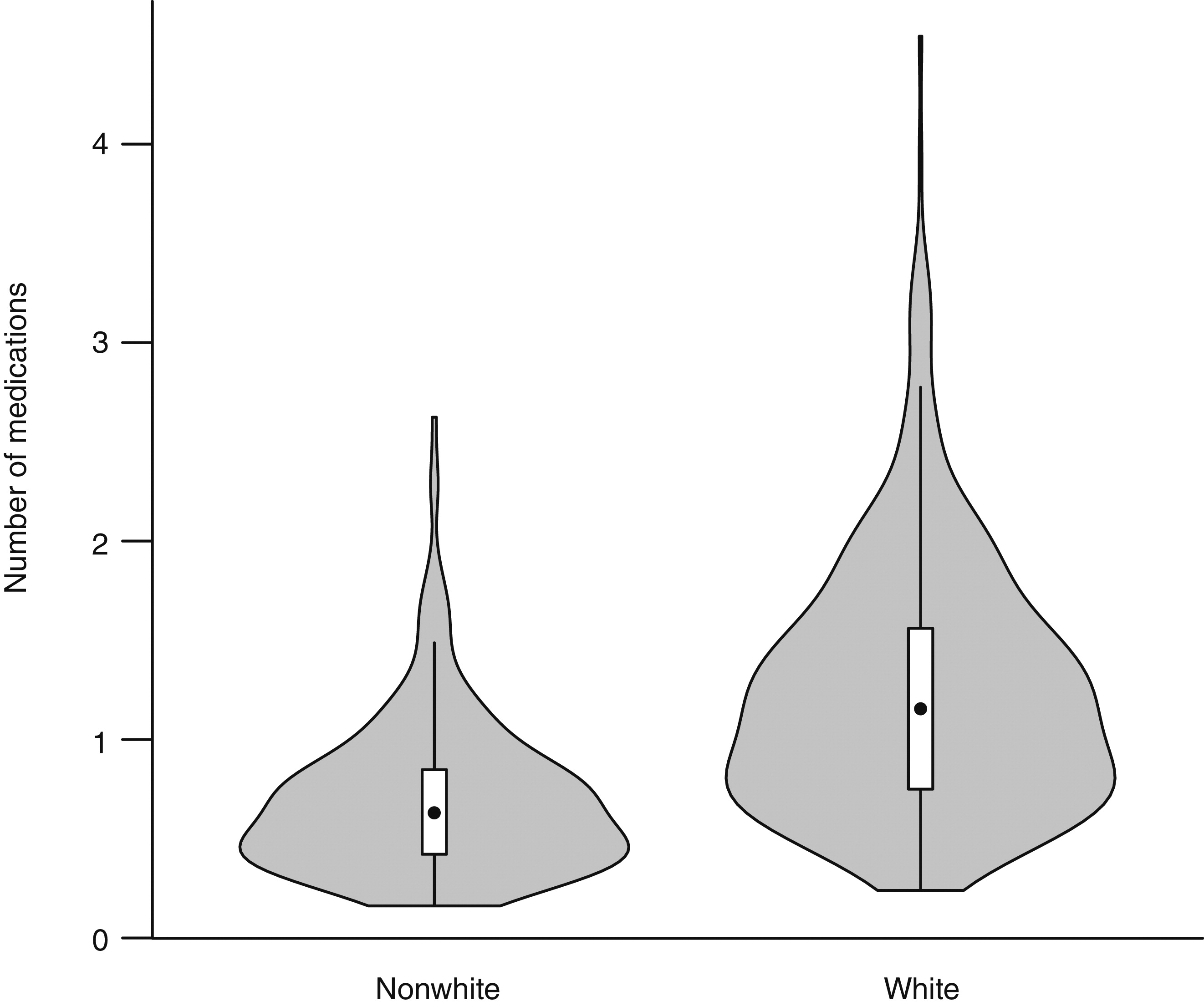
4 summarize the results. Apparent site differences in the rates of antipsychotic, mood stabilizer, and antidepressant use were no longer significant after adjustment for demographic, clinical, and provider variables. No initial site differences in stimulant rates were found. The block of demographic variables significantly predicted probability of usage of all four medication classes; however, white race was the only demographic variable to provide significant unique incremental information at all subsequent blocks of the models for antipsychotics, mood stabilizers, and antidepressants. Even when the analysis controlled for all other variables, the odds of white youths receiving each of these three medication classes were three times higher than for other youths with similar insurance, family histories, diagnoses, severity of presentation, and access to professional providers. The white youths were significantly more likely to have taken an antipsychotic in the past (r=.15, p<.001) and a stimulant in the past (r=.13, p<.01) (
Table 4 and
Figure 1). After control for other variables, no differences by race in current stimulant usage were found. Sensitivity analyses found that excluding the 18 youths who were seeking only evaluation and not taking medication did not change the results.
Discussion
This study examined medication practice patterns in the carefully ascertained baseline LAMS sample of 698 children with mood and behavior problems. The findings suggest polypharmacy among just over one-fourth of children enrolled at their first outpatient child mental health clinic visit. Among those who were receiving more than one medication, an additional agent was often added to stimulant monotherapy for ADHD. Just over one-third (38%) of participants were not treated with any psychotropic agents, even though most had one or more psychiatric disorders. A substantial proportion of children with ADHD were not receiving a stimulant or any other psychotropic treatment.
The finding that a majority of participants with bipolar disorder were treated with antipsychotics, mood stabilizers, or both is consistent with practice patterns in other areas of the country (
23,
24) and with current treatment guidelines for pediatric bipolar disorder (
25). Participants without bipolar disorder were treated mainly with stimulant medications. Unlike other studies, this study found no evidence that minority race (
26–
28) or Medicaid status (
26) led to a greater likelihood of being prescribed psychotropic medications. Instead, the odds of polypharmacy were three times higher among white youths than among nonwhite youths, even after the analysis controlled for other demographic, insurance, and clinical characteristics. This result contradicts some findings from prior claims-based studies (
29) but is similar to findings of clinical studies (
22,
30–
33).
Our findings of increased psychiatric medication use among whites suggest that other factors are at work. One possibility is differences in beliefs about the causes of mental illness (
34,
35); another is differences in attitudes toward pharmacological treatment (
36). Other investigations have found that white middle-class families tend to subscribe more often than other racial or ethnic groups to biological explanations of mental illness, and they also tend to have more favorable attitudes toward medication as a component of treatment (
37,
38). This study did not directly measure participants’ attitudes and beliefs; however, if they were consistent with those of participants in previous studies, this would help explain the robust difference in rates of polypharmacy observed in this study. A third possibility is that white participants had more actively sought interventions (for example, from their primary care physician) before arriving at the child mental health clinics from which they were ascertained. This scenario is possible because the analyses indicated that white youths in this sample were significantly more likely to have taken an antipsychotic or stimulant in the past.
This study had several limitations. First, although 79% of eligible families agreed to complete the screening instrument, only 56% of those eligible at screening agreed to participate in the longitudinal portion of the study. Therefore, generalizability of these data may be limited. Second, data on medication usage were based on parent report and were not verified via another source, such as by monitoring prescription fills or by pill counts. Third, because the sample included children who were experiencing elevated symptoms of mania, service utilization may be overestimated compared with general outpatient samples. Finally, these data were cross-sectional, and reports of medication use are a mixture of prescriptions before the mental health clinic visit and prescriptions by the mental health clinic between screening and baseline assessment. However, results remained robust when the analysis excluded participants who were seeking only evaluation and not taking medication. Identification of patterns and predictors of service use and outcomes and predictors of improved outcomes will require data from the longitudinal portion of the LAMS study, which is currently in process.
Conclusions
These results contrast with findings from some studies of large pharmacoepidemiologic claims–based data sets (
6,
39–
41) that suggested that psychotropic medications are overprescribed to children and adolescents. Results from pharmacoepidemiology studies are often limited by selection bias, information bias, confounding, and lack of clear group diagnostic validity and reliability (
42,
43). The LAMS study design complements claims-based research by ascertaining a clearly defined group of cases and then using semistructured diagnostic interviews to determine diagnoses and service utilization. In the LAMS sample, when medications were prescribed, they appeared linked meaningfully to diagnoses and functioning in ways broadly consistent with evidence-based practices and with rational approaches to intervention in the community. The LAMS study is continuing for a second five years, and in the near future we will be able to examine the effects of psychiatric medication treatment—and nontreatment—on the clinical outcomes of youths in this sample.
Acknowledgments and disclosures
The LAMS study is funded by grant MH073816 from the National Institute of Mental Health.
Dr. Kowatch is a consultant for Forest Pharmaceuticals, AstraZeneca, and the REACH Foundation. Dr. Youngstrom has served as a consultant for Lundbeck and has received travel support from Bristol-Myers Squibb. Dr. Frazier has received funding or research or travel support from, served as a consultant to, or received a speaker’s honorarium from Forest Laboratories, Ecoeos, IntegraGen, Shire, and Bristol-Myers Squibb. Dr. Arnold has received research funding, advisory board honoraria, or travel support from Biomarin, Curemark, Eli Lilly and Company, Forest Laboratories, Novartis, Noven, Roche, Seaside Therapeutics, and Shire; and has served as a consultant for Tris Pharma. Dr. Findling receives or has received research support from, acted as a consultant to, or served on a speaker's bureau for Alexza Pharmaceuticals, AstraZeneca, Bracket, Bristol-Myers Squibb, Clinsys, Cognition Group, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, KemPharm, Lundbeck, Merck, Novartis, Noven, Otsuka, Pfizer, Rhodes Pharmaceuticals, Roche, Seaside Pharmaceuticals, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. The other authors report no competing interests.