Cost-Effectiveness of Esketamine Nasal Spray for Patients With Treatment-Resistant Depression in the United States
Abstract
Objective:
Methods:
Results:
Conclusions:
HIGHLIGHTS
Methods
Overview
Model Description
Model Input Data
| Parameter | Base case value | Uncertainty analysis range | Distribution | Source |
|---|---|---|---|---|
| General and demographic | ||||
| Annual discount rate (%) | 3 | Sanders et al. (17) | ||
| Time horizon (years) | 5 | 2–10a | ||
| Annual mortality probability (%) | .40 | .38–.43 | Normal | Amos et al. (25), Arias et al. (26), Cuijpers et al. (27) |
| Esketamine efficacy relative to usual care | Janssen (4) | |||
| Response (relative risk) | 1.32 | 1.10–1.58 | Log-normal | |
| Remission (relative risk) | 1.39 | 1.09–1.79 | Log-normal | |
| Relapse (hazard ratio) | 1 | |||
| Usual care efficacy by treatment line | Rush et al. (7) | |||
| Remission probability (%) | ||||
| Line 3 | 30.6 | 28.2–33.0 | Beta | |
| Line 4 | 13.7 | 10.4–17.2 | Beta | |
| Lines 5+ | 13.0 | 7.7–19.5 | Beta | |
| Response probability (%) | ||||
| Line 3 | 28.5 | 26.2–30.9 | Beta | |
| Line 4 | 16.8 | 13.4–20.8 | Beta | |
| Lines 5+ | 16.3 | 10.3–23.2 | Beta | |
| Monthly relapse probability (%) | ||||
| In remission, line 3 | 7.8 | 6.6–9.1 | Beta | |
| In remission, line 4 | 7.7 | 4.1–12.6 | Beta | |
| In remission, lines 5+ | 8.6 | 3.7–16.4 | Beta | |
| In response, line 3 | 13.6 | 11.4–16.1 | Beta | |
| In response, line 4 | 17.8 | 12.9–24.2 | Beta | |
| In response, lines 5+ | 17.3 | 11.0–27.4 | Beta | |
| Utility by health state | Sapin et al. (30) | |||
| Nonresponse, relapse, initiation | .58 | .50–.66 | Normal | |
| Response | .72 | .65–.79 | Normal | |
| Remission | .85 | .83–.87 | Normal | |
| Costs (2015 $) | ||||
| Annual direct health care cost by treatment line | Amos et al. (25) | |||
| Line 3 | 12,047 | 9,666–14,428 | Normal | |
| Line 4 | 14,699 | 11,441–17,957 | Normal | |
| Line 5 | 15,073 | 13,053–17,093 | Normal | |
| Line 6 | 16,699 | 13,939–19,459 | Normal | |
| Lines 7+ | 18,667 | 17,089–20,245 | Normal | |
| Annual lost productivity cost by health state | Stewart et al. (38) and Bureau of Labor Statistics (39) | |||
| Nonresponse, relapse, initiation | 11,573 | 8,063–15,083 | Normal | |
| Response | 5,757 | 3,445–8,070 | Normal | |
| Remission | 2,067 | 1,338–2,796 | Normal | |
| Monthly costs of esketamine treatment | ||||
| Medication costs | Janssen (4) and U.S. Department of Veterans Affairs (34) | |||
| Month 1 | 5,572 | |||
| Months 2+, response | 2,244 | |||
| Months 2+, remission | 1,699 | |||
| Months 2+, nonresponse, relapse | 1,966 | |||
| Physician and medical assistant services | Janssen (4), Centers for Medicare and Medicaid Services (36), and Bureau of Labor Statistics (37) | |||
| Month 1 | 685 | |||
| Months 2+, response | 275 | |||
| Months 2+, remission | 218 | |||
| Months 2+, nonresponse, relapse | 246 | |||
| Patient time | Janssen (4) and Bureau of Labor Statistics (39) | |||
| Month 1 | 689 | |||
| Months 2+, response | 276 | |||
| Months 2+, remission | 219 | |||
| Months 2+, nonresponse, relapse | 248 |
Demographics and mortality.
Esketamine efficacy.
Usual care efficacy.
Utility.
Costs.
Uncertainty and Sensitivity Analyses
Results
Model Validation
Five-Year Health and Economic Outcomes
| Parameter | Usual care | Esketamine | Difference |
|---|---|---|---|
| Time spent in health state (%) | |||
| Initiation, nonresponse, relapse | 72.3 | 66.9 | –5.4 |
| Response | 2.4 | 2.0 | −.4 |
| Remission | 25.3 | 31.1 | 5.7 |
| QALYsa | 3.00 | 3.07 | .07 |
| Total costs (2015 $) | |||
| Societal perspective | 121,073 | 137,690 | 16,617 |
| Health care sector perspective | 79,786 | 96,781 | 16,995 |
| Cost components (2015 $) | |||
| Esketamine treatment | |||
| Medication | 16,352 | 16,352 | |
| Physician and medical assistant services | 2,062 | 2,062 | |
| Patient time | 2,074 | 2,074 | |
| Other health care costs | 79,786 | 78,367 | –1,419 |
| Lost productivity | 41,287 | 38,836 | –2,451 |
| Incremental cost-effectiveness ratios ($/QALY) | |||
| Societal perspective | 237,111 | ||
| Health care sector perspective | 242,496 |
Uncertainty and Sensitivity Analyses
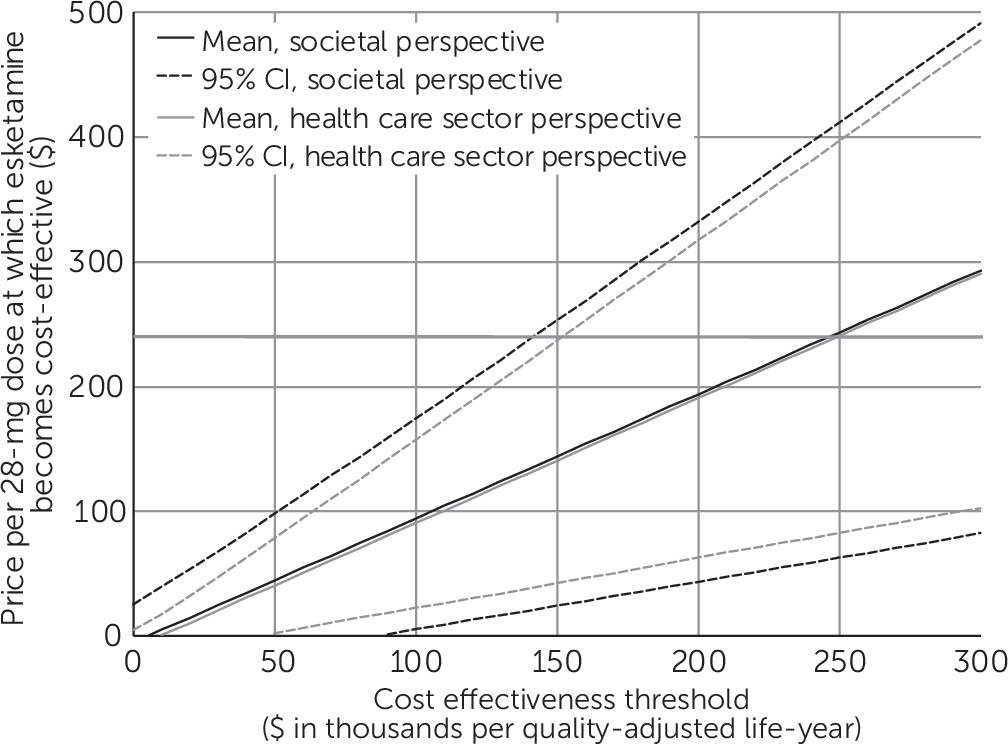
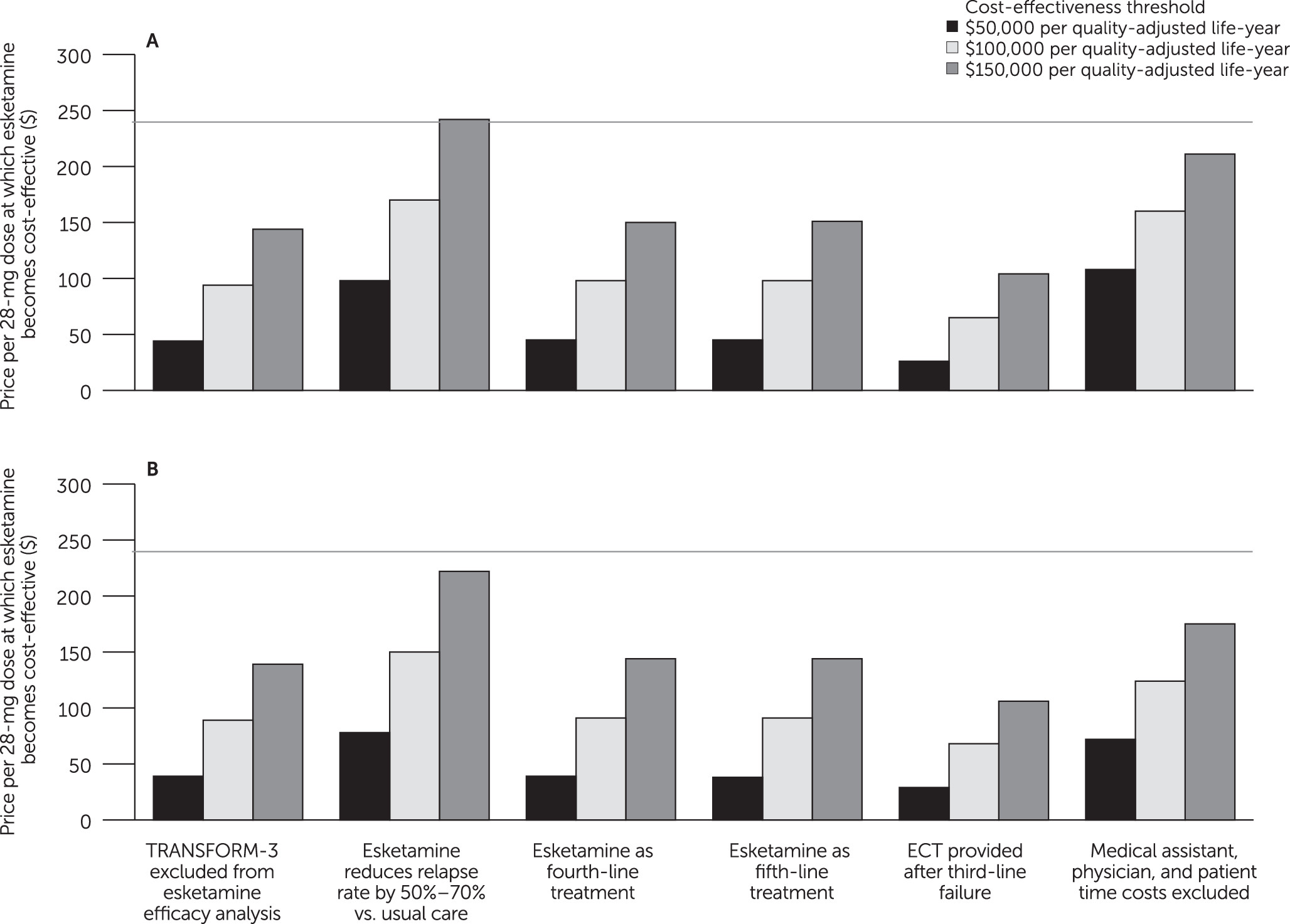
Discussion
Conclusions
Supplementary Material
- View/Download
- 417.43 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

