Major depressive disorder causes substantial increases in morbidity and mortality rates across the life span. It increases the risk for suicide, increases health care utilization, and, among patients ages <50 years, contributes to more disability-adjusted life years lost than does diabetes, stroke, heart disease, or hypertension. Although many patients respond to first-line treatments (i.e., antidepressant medications and psychotherapy), accumulating evidence suggests that individuals who are socioeconomically disadvantaged—including those who are unemployed, living in poverty, and having lower income and educational attainment—manifest worse mental health outcomes than do people of other socioeconomic strata (
1).
Historically, research has focused on the hypothesis that socioeconomic status affects mental health outcomes through its impact on access to health care, provider availability, cost of treatment, and engagement with the health care system (
2,
3). These studies have suggested that the socioeconomic environment of patients with major depressive disorder directly affects outcomes of treatments for this disorder. However, this hypothesis is difficult to assess through examination of observational data, because variation in treatment access, provider availability, cost, and engagement are generally difficult to measure and are often correlated with other important factors, such as financial barriers, health insurance coverage, income, and quality of care, that can confound findings (
4,
5). Randomized controlled trials (RCTs) allow examination of socioeconomic factors by controlling for related variables, such as access and quality of care, in analyses (
6).
The Combining Medications to Enhance Depression Outcomes (CO-MED; [
7]) study provides a means by which socioeconomic variables can be examined across a large heterogeneous sample. Patients (N=665 adults, ages 18–75 years) enrolled in CO-MED received one of three antidepressant treatments for 12 weeks. The primary outcomes of the CO-MED trial have been described previously; however, the impact of socioeconomic factors—other than race-ethnicity—on treatment outcomes has received limited attention (
8,
9) in prospective RCTs. Furthermore, the trajectory of treatment responses with regard to these features has received almost no attention. All CO-MED patients had access to the same treatments, which were provided free of charge and by the same providers working in academic health centers, thereby potentially eliminating any differential effects of financial barriers, health insurance coverage, and provider quality.
With this in mind, we used Bayesian hierarchical models (
10,
11) to examine the impacts of educational achievement, income, employment status, and race-ethnicity on treatment responses (trajectory) among adults with major depressive disorder, while controlling for clinical variables, such as access to and quality of care. We hypothesized, on the basis of accumulating data from the socioeconomic disparities’ literature, that income, unemployment, lack of a college education, and being a member of a minority group would each be associated with reduced improvement in depressive symptoms, even after eliminating the effects of differential treatment access and quality of care.
Methods
As previously described (
7), outpatients with nonpsychotic chronic or recurrent major depressive disorder were recruited from six primary and nine psychiatric care sites and randomly assigned (at a 1:1:1 ratio) to receive escitalopram plus a placebo, escitalopram plus bupropion, or venlafaxine plus mirtazapine. The inclusion criteria were broad, with only few exclusion criteria (
7). Patients had to have had an index depressive episode for ≥2 months and a score of ≥16 on the 17-item Hamilton Depression Rating Scale (HAM-D) (
7). The protocol and all consent and study procedures were approved by the institutional review boards at the National Coordinating Center (University of Texas Southwestern Medical Center) and all clinical sites.
The study was conducted from March 2008 to April 2014. Treatment visits for the acute phase examined in this study were at baseline (week 0) and weeks 4, 8, and 12, and medication dosage was adjusted in response to scores on the 16-item Quick Inventory of Depressive Symptomatology–Clinician-Rated (QIDS-SR) (
7).
The individual trajectory of improvement (i.e., weeks 0, 4, 8, 12) in depressive symptoms (as measured by the QIDS-SR) was modeled as an individual logarithmic trends “random effects” coefficients Bayesian hierarchical model (
12). The multilevel Bayesian hierarchical model has several advantages over the approach of using a standard mixed model for repeated measures—most notably allowing exact posterior inference and explicit modeling and estimation of both observed and unobserved heterogeneities—and provides improved inference and precision (
10–
12). Several functional forms for the trend (linear, time indicator fixed effects, log-linear, linear-log, and double-log) were compared with Akaike information criterion and Bayesian information criterion and predictive performance, and, as observed in several previous studies of psychopharmacological treatment efficacy, the linear-log trend model was overall the best specification (
12).
For the estimated trajectory models, the indicator dummy variables for each predictor took values of 0 or 1, so model-predicted values with the indicator variable (e.g., employment) being present (value of 1) or absent (value of 0) provided conditional predictions based on the presence or not of this characteristic. Because income was a continuous variable, we used the sample income values at the 25th and 75th percentiles to generate conditional predictions at the model end point (week 12). The predicted values at the end point were then used to compute percentage differences in improvement. The number of observations was thus the same (i.e., used the full sample) for each prediction. (Specific details of the model are provided in the
online supplement to this article.)
Results
Demographic and clinical characteristics of the patients, including race-ethnicity and income, are reported in Table S1 of the
online supplement and have been previously described (
7). Briefly, patients with a college degree–level education (>16 years of schooling) had baseline depression symptom severity similar to that of those with less education (<16 years of schooling) (mean QIDS-SR=15.3 vs. 15.6, p=0.956). Similarly, age (42.3 vs. 43.7 years, p=0.937) and level of anxiety (mean HAM-D score=7.9 vs. 8.4, p=0.886) were similar for those with and without a college degree, respectively. Those who were employed had similar baseline depression symptom severity (15.1 vs. 15.92, p=0.888), age (41.4 vs. 45.3, p=0.830), and anxiety levels (8.1 vs. 8.4, p=0.920) compared with those who were unemployed.
In the whole sample (N=569 available for estimation after patient visits with missing values were omitted), in an analysis that controlled for age, sex, baseline symptom severity, and presence of anxiety, the mean±SD log trend coefficient provided significant evidence of overall improvement in depression symptoms (β=−0.489±0.018, credible interval [CrI]=−0.455 to −0.524, p<0.001) (see Table S2 of the
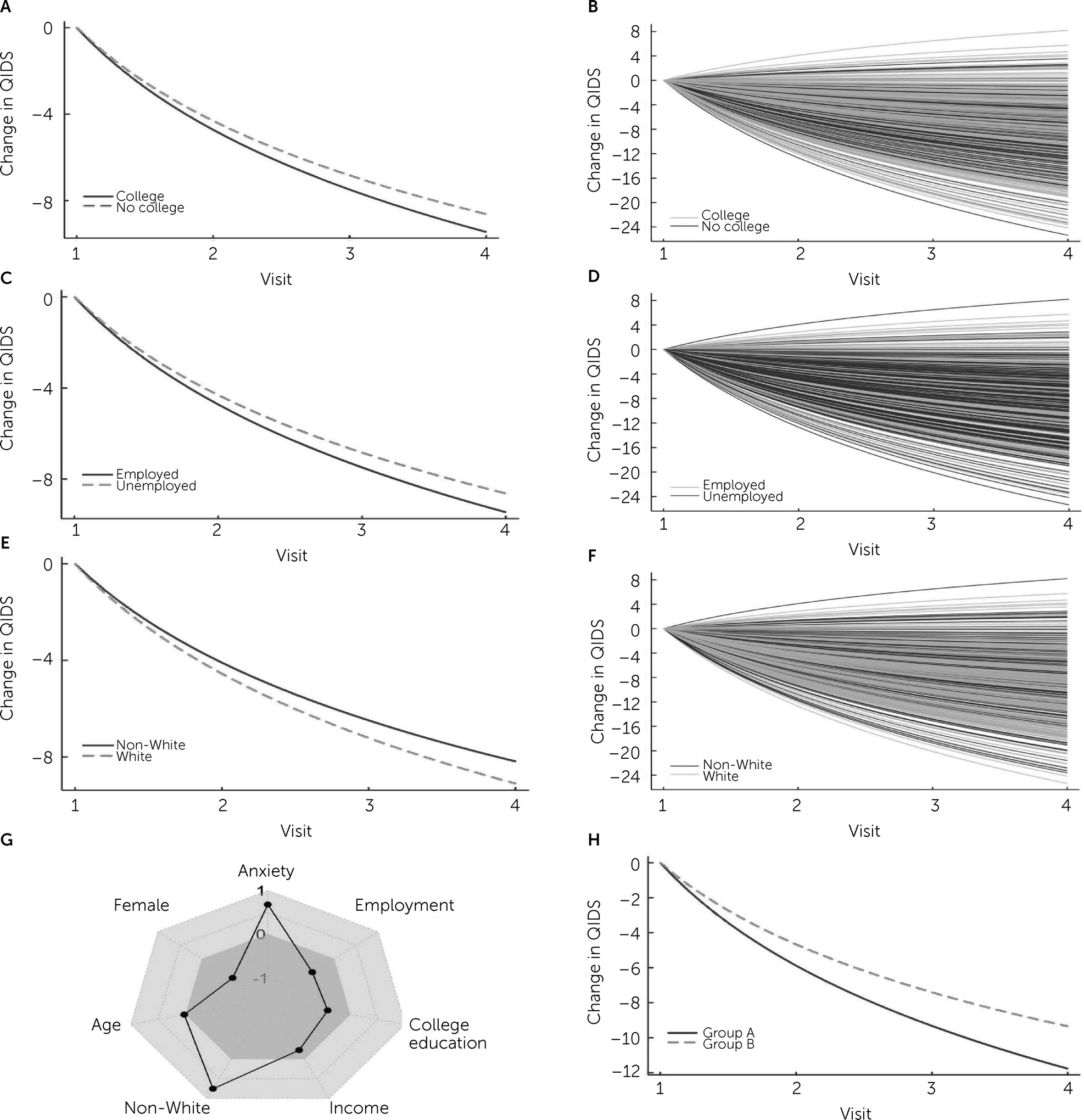
online supplement). Note that a negative coefficient estimate indicates a declining QIDS-SR and so improvement over time. Not having a college education (β=0.410±0.205, CrI=0.009–0.811, p=0.045), being unemployed (β=0.490±0.182, CrI=0.133–0.848, p=0.007), and being non-White (β=0.753±0.193, CrI=0.374–1.131, p<0.001) all had positive coefficients and so were associated with slower and less improvement (
Figure 1; also see Table S2 of the
online supplement). Additionally, improvement in depression symptoms was significantly correlated with (standardized) income (β=−0.225±0.095, CrI=−0.038 to −0.412, p=0.019), with lower income being associated with less improvement (
Figure 1; also see Table S2 of the
online supplement).
On the basis of conditional predicted values generated from the estimated trajectory models (see Methods and Table S2 in the
online supplement; N=569), being unemployed resulted in 6.6% less improvement at the treatment end point (week 12, p=0.007), not having a college degree led to 9.6% less improvement (p=0.045), being non-White led to 11.3% less improvement (p<0.001), and having an income at the 25th percentile of the income distribution resulted in 4.8% less improvement than having an income at the 75th percentile (p=0.018). (Percentages were generated by using the estimated coefficients from the same models used to generate
Figure 1 and models similar to that provided in Table S2 in the
online supplement.) Because these characteristics were likely associated with each other (e.g., because people with lower income are less likely to have a college education), we examined the impact of multiple negative influences on treatment outcomes. A comparison of outcome trajectories for patients who were White, employed, had a college degree, and had an income at the 75th percentile with those who were non-White, unemployed, had no college degree, and had an income at the 25th percentile indicated that the latter group had 25.9% less improvement at the end of treatment (see
Figure 1, Panel H).
Discussion
The social determinants of health outcomes have been well described, and recent calls have directed clinicians’ attention to the need for improving access to health care, greater affordability of treatment, and reducing language barriers and other constraints (
13). However, although previous work has acknowledged that these social inequality–driven structural factors collectively produce disproportionate outcomes for some individuals, most of this work has focused on the naturalistic effects of these factors. Observation of these effects within clinical trials that control for several of these factors—namely, health care access, treatment costs, provider availability, and engagement with the health care system—has important clinical implications and also implications for researchers and policy makers. Our findings show that employment, education, race-ethnicity, and income have significant effects on treatment responses and that these effects are independent of access to care, cost of medication, insurance coverage, and quality of care. How much the symptoms of a patient with major depressive disorder improve within a clinical trial has long been thought to relate to the type of medication being administered, as well as to the medication’s pharmacokinetics and pharmacodynamics. In some trials, clinical factors (e.g., baseline symptom severity and comorbid conditions) affect treatment responses. Our results suggest that a significant amount of variation in the treatment response relates to patients’ socioeconomic characteristics. These findings have implications for public policy, clinical trial design, and individualized approaches to treatment.
From a public policy perspective, approaches to treating depression are frequently siloed and rarely consider the patient’s socioeconomic milieu (
14). Our findings raise the possibility that attending to socioeconomic factors could enhance outcomes for a substantial number of patients and that outcomes may be independent of the specific psychopharmacologic treatment. Historically, the effects of socioeconomic adversity on treatment outcome have been attributed to decreases in access to care and to the quality of care received. However, the influence of care quality and access are generally controlled for in an RCT, highlighting that socioeconomic differences in outcomes can arise from other factors related to patients’ environments.
When interventions—whether psychosocial or pharmacologic—are evaluated in RCTs, variation in patients’ responses to the interventions is generally attributed to differences in the interventions’ efficacy. However, our findings suggest that substantial variation in responses results from socioeconomic factors. These factors are infrequently assessed in many clinical trials and are rarely considered in outcome models. The analyses of most clinical trials have included clinical characteristics (e.g., baseline symptom severity and comorbid conditions) and demographic characteristics (e.g., age and sex) rather than socioeconomic factors. Traditional analyses of these trials often assume that socioeconomic variables are “controlled for,” given that there is universal access to care and that other barriers to treatment are removed by the structure of the RCT. Thus, not only measuring but incorporating these factors into analyses of RCTs could enhance our ability to detect treatment-placebo differences or differences among active treatments. Additionally, consideration of socioeconomic factors could decrease the likelihood of abandoning effective treatments because of socioeconomically driven influences and could further enhance our understanding of patient-specific trajectories of improvement and could help create targeted therapies.
Very few studies have examined socioeconomic factors that may influence improvement among adults with depressive disorders in the context of a prospective RCT, and several limitations of this study warrant discussion. First, many of the socioeconomic factors likely represented proxies for other features that are difficult to measure. For example, patients with less education experienced slower and less improvement; however, this result may have been related to multiple factors (
15). Those with less education may attribute or express symptoms and improvement differently from those who could pursue more education. Furthermore, individuals with less education, even when employed, may face lower job security and more stressful job conditions, which could attenuate improvement in depression treatment. Second, our results were based on a deterministic logarithmic trend model, which imposed a more restrictive functional form for the trajectory of response but improved our precision. Third, we were limited in our ability to examine all moderators of treatment responses, and, despite the impressive sample size of CO-MED, we had a limited number of observations over time.
Conclusions
In summary, the findings of this study reveal the impact of social determinants on treatment outcomes in depression. Future clinical trials should measure these socioeconomic factors and incorporate them analytically. Additionally, to optimize treatment outcomes for adults with depression, we must leverage socioeconomic variables as potential predictors of outcomes to inform treatment selection, monitoring, and delivery.
Acknowledgments
The authors acknowledge the administrative support of the Research and Development Service at the participating VA Medical Centers.