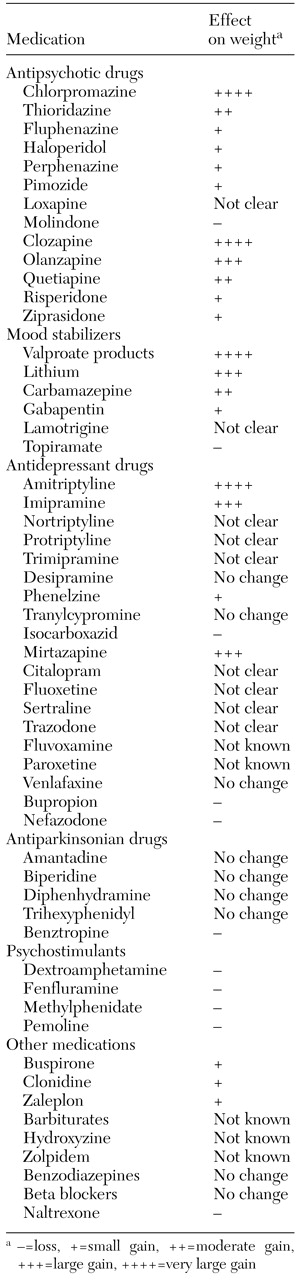
Antipsychotic drugs
Each of the antipsychotic agents differs in terms of the timing and severity of potential weight gain. Increased body mass is especially problematic with the long-term use of low-potency conventional neuroleptics and the atypical agents clozapine and olanzapine (
9,
10,
11), as indicated in
Table 1.
Conventional neuroleptics. Weight gain associated with chlorpromazine and thioridazine has been well described (
8,
10). In one study, patients who were treated with chlorpromazine increased their food consumption and gained an average of 4 kg during the course of three months of therapy (
10). Other research showed an average increase of 16 percent over maximal ideal body weight (
12). High-potency neuroleptics, such as fluphenazine, have less of a weight-promoting effect than low-potency neuroleptics (
8). A 10 percent weight gain over the course of a year was reported among 19 patients who were treated with pimozide or fluphenazine (
13). Perphenazine may result in some weight gain; however, there is no evidence of this effect for haloperidol (
8). Loxapine has been associated with both losses and gains.
The mechanism of neuroleptic-associated weight gain is related to blockage of anticholinergic, serotonergic, and histaminergic sites, all of which are related to appetite stimulation (
8). Haloperidol has a low affinity for such receptors, which may explain its low potential for inducing weight gain (
10,
14).
Studies of the effects of molindone are controversial. An average weight loss of 4.5 kg has been observed, and some studies have documented a neutral effect (
10,
15,
16). An eight-week trial showed a mean±SD reduction of 2.2±3.7 kg among patients who had been treated with molindone (
15). The weight-decreasing properties of molindone have been attributed to an anorectic effect, the drug's ability to increase motor activity, and lack of alpha
1-adrenergic and histamine-receptor blocking properties (
15,
16).
Atypical antipsychotic drugs. Atypical antipsychotic drugs are associated with fewer side effects. However, these agents can also cause weight gain and may adversely affect glucose metabolism, with a diabetogenic influence.
Clozapine has been strongly associated with weight gain (
10). A retrospective study showed that patients gained an average of 8 kg while taking clozapine (
17). The accumulations were substantial and exceeded those associated with the use of conventional neuroleptics. Patients gained most of their weight during the early months of drug treatment and then continued to gain at a slower rate for several years (
18,
19).
Clozapine's mechanism of action includes antagonism at serotonin 5-HT
2 receptors, activity at alpha-adrenergic receptors, and influence at the histaminergic, cholinergic, endocrine, and metabolic systems (
17,
20). Clozapine may impair glucose homeostasis through altered secretion or utilization of insulin and growth hormone, which is mediated by serotonin and histamine receptors (
21,
22). Abnormal glucose tolerance has been documented among patients being treated with clozapine (
23).
Olanzapine has been shown to have a potent effect on weight gain; up to 94 percent of persons who take olanzapine may experience this side effect (
9,
24). Several studies found that patients who were receiving long-term treatment with olanzapine gained an average of 12 kg during the course of one year (
25,
26,
27,
28). However, in a retrospective study, 100 patients gained an average of only 2 kg after taking olanzapine for six months (
14). Recently collected data point to significant weight gain among patients taking olanzapine (
29). The drug's mechanism of action involves blocking activity at serotonergic 5-HT
2, muscarinic, cholinergic, histaminic, alpha-adrenergic, and dopamine D
1 and D
2 receptors (
20).
Risperidone has been associated with minimal to moderate weight gain. In a recent study of patients with schizophrenia who were taking either olanzapine or risperidone, no significant change in mean body weight was observed (
29). A retrospective study showed that patients who were treated with risperidone for a mean period of 112 weeks gained an average of 1.7 kg (
27). In a European multinational comparison study of risperidone, weight gain was observed among 26 to 38 percent of treated patients in a dose-dependent correlation (
30). Long-term treatment with risperidone has been shown to increase patients' tendency to gain weight (
22,
31).
The time frame for weight gain remains controversial. In one study, patients who were taking risperidone experienced no change in body mass index after several months of treatment (
28), whereas patients taking risperidone in another trial gained weight over several weeks (
32). Risperidone is a combined dopamine D
2 and serotonin 5-HT
2 antagonist, which may explain its effect on body mass (
20,
28).
Quetiapine has been associated with small increases in weight. In clinical trials of quetiapine, the average weight gain was 1 to 4 kg during the initial months of treatment (
33). Although continued gains have not been documented, a 7 percent increase was reported for 25 percent of quetiapine-treated patients in one study (
34). The drug acts mainly at histaminic and alpha
1- and alpha
2-adrenergic receptors (
20,
27).
Ziprasidone is the most recently released of the atypical antipsychotics. It also appears to be the one associated with the smallest weight gains (
10).
Mood stabilizers
Most mood stabilizers, such as lithium, valproic acid derivatives, carbamazepine, gabapentin, and lamotrigine, can cause weight gain (
8,
24), as shown in
Table 1. The valproate-related products are the biggest offenders. Topiramate, however, has been associated with weight loss (
35,
36).
Lithium. Weight gain is a frequent side effect of long-term maintenance therapy with lithium (
7,
27,
37,
38). A mean gain of 10 kg was reported for 46 of 70 patients who had been taking lithium for two to six years (
37). Those who were obese at the start of the study were more prone to increases. In another investigation, involving 21 patients who were treated with lithium, 11 gained more than 4 kg (
38). In a sample of patients who were taking lithium over a ten-year period, 11 to 65 percent gained an average of 10 kg; gains of up to 27 kg have been reported with protracted exposure in a dose-dependent pattern (
27).
The mechanism for lithium-induced weight gain without hypothyroidism is not known. Several explanations have been proposed. Lithium often increases thirst and may foster consumption of high-calorie liquids (
37). Lithium-induced edema may also be a contributor (
8). Controversial data suggest that lithium increases storage of carbohydrates and lipids (
39,
40,
41). Lithium-induced hypothyroidism could also explain weight accumulations (
42).
Valproate-related agents. Among the mood stabilizers, valproic acid and its derivatives are associated with significant weight gain, at an average of 8 to 14 kg and an incidence of 8 to 59 percent (
43). Of 63 adults who were treated with valproic acid, 36 gained more than 4 kg during pharmacotherapy, and weight was stable for 27. Weight gain may be explained by increased food intake, decreased energy expenditure, reduction of thermogenesis, and greater availability of long-chain fatty acids as a result of competitive binding to serum albumin (
42,
43,
44). Valproate-related fat deposition is difficult to reverse with dietary restrictions (
44). Valproic acid may increase serum leptin concentrations; such increases have also been associated with increases in body mass (
45).
Carbamazepine. Carbamazepine is less commonly associated with weight gain. In a sample of 24 patients, significant gains were documented among those who were depressed (
46). In a subgroup of patients with bipolar disorder, no significant changes were noted. It is possible that weight gain is related more to improvement in mood than to a direct effect of the drug (
47). Information about appetite stimulation is controversial. Weight gain combined with little appetite stimulation was documented among patients who switched from lithium to carbamazepine (
2). Another study demonstrated an abrupt increase in both appetite and weight after patients started taking carbamazepine (
47).
Topiramate. In contrast, topiramate can induce weight loss. Among 40 people for whom topiramate was used to supplement other antiepileptic drugs, the average reduction was 3 to 8 kg (
35). Another report described experiences with topiramate for two obese patients (
36). The substitution of topiramate for valproate was associated with a loss of 7 to 11 kg while the patients were taking topiramate at a dosage of 200 to 300 mg a day (
36). Topiramate-associated weight change appears to be dose related. In several trials, the mean decrease ranged from 1.6 kg in a low-dosage group to 6.5 kg in the group that received the highest dosage (
48).
Topiramate has effects on gamma-aminobutyric acid function in the hypothalamus, but the precise mechanism of its influence on body mass remains unknown (
49). Some patients who were treated with topiramate reported a loss of appetite associated with altered taste, which may have been due to the inhibitory action of topiramate on carbonic anhydrase (
42).
Other agents. Although gabapentin, lamotrigine, and tiagabine have generated interest as possible mood stabilizers, clinical experience with their use is limited, and their potential for causing weight change is unclear. A 3 percent incidence of weight gain was observed among patients who were taking gabapentin at daily doses of 900 to 1,800 mg (
42). Data on lamotrigine show no consistent alterations of clinical significance (
50).
Antidepressants
Most antidepressants are associated with increased weight, as
Table 1 shows. Antidepressants can enhance appetite and induce a craving for carbohydrates (
8). This tendency of antidepressants to cause weight gain may be related to improved appetite and more joyful eating patterns as symptoms of depression diminish. The mechanism of action and associated biochemical alterations, such as of leptin or tumor necrosis factors, are being investigated (
51). Tricyclic antidepressants cause weight gain more often than do monoamine oxidase inhibitors. New-generation antidepressants have been associated with various patterns of change.
Tricyclics. Tricyclic drugs have been implicated in weight gain. Anticholinergic activity has been considered a possible mechanism for weight gain in that it causes dry mouth, which can lead to excessive consumption of high-calorie beverages. A craving for sweets has been reported among patients taking amitriptyline, nortriptyline, and imipramine (
5). Blockage of norepinephrine, dopamine, and serotonin from the presynaptic terminal and interactions with muscarinic, cholinergic, alpha1-adrenergic, and histaminic receptors characterize the side effect profiles of tricyclic medications.
The effects of amitriptyline on body mass have been extensively investigated. In one study involving 51 women who were taking amitriptyline, the average weight gain was 4 kg (
8). In another study, the average gain was 7 kg, and 73 percent of the study participants reported an increase in their desire for sweets (
52). Amitriptyline has been associated with weight gain in samples of patients who ranged from underweight to obese (
53).
Imipramine was studied in a 16-week weight-monitoring trial of 53 persons with depression. An average gain of 2 kg was noted, and 15 percent of the study participants gained more than 6 kg (
54). In a prospective double-blind study, persons who were taking imipramine tended to have a greater desire to eat but did not experience any weight changes (
52).
Desipramine is less problematic. In an open, five-week study of 41 desipramine-treated patients, no alterations in weight were observed (
55). However, this lack of association remains controversial. Desipramine may be a valuable—but unproven—treatment alternative when weight gain is of concern because of its low potency at the histamine H
1 receptor and because it has few anticholinergic effects.
In a 30-week trial of nortriptyline, seven of 29 participants (24 percent) lost weight (
56). Sixteen participants (55 percent) gained weight, but the increase was considered clinically relevant for only five of them. In another study, the average gain was up to 4 kg (
8). Other tricyclic medications, such as protriptyline and trimipramine, have been associated with weight loss or gain (
57). Data on the effects of amoxapine are limited.
Monoamine oxidase inhibitors. Monoamine oxidase inhibitors (MAOIs) are less likely to produce weight gain than are tricyclic antidepressants. Within the MAOI group, phenelzine is more likely than tranylcypromine to induce weight gain as a result of associated carbohydrate craving and edema (
57,
58). No cases of tranylcypromine-induced gains have been reported. Loss of appetite and of weight may occur with isocarboxazid (
58). Different MAOIs have different effects on appetite control.
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) selectively block the reuptake of serotonin into presynaptic nerve terminals. Because they enhance serotonin, it is possible that use of SSRIs results in a lower intake of carbohydrates. Minimal weight gain and decreases in appetite have been associated with the use of SSRIs (
59). In fact, fluoxetine and sertraline may have potential as therapy for obesity (
60). Although there are few data on weight changes during treatment with fluvoxamine or paroxetine, anecdotal evidence indicates that the latter agent can precipitate weight gain (
61). The effects of SSRIs on weight have not been systematically explored.
Fluoxetine was not associated with weight gain in a placebo-controlled trial with 832 patients who were receiving long-term therapy; rather, an acute-phase loss of .35 kg was reported (
62). After 38 weeks, patients in the treatment group gained up to 2 kg, compared with 2.5 kg in the placebo group. Some gains were observed in a one-year study of the effects of fluoxetine (
63).
Citalopram may not produce significant changes in body mass (
57). In a placebo-controlled trial of short-term treatment that included more than 1,000 patients, citalopram was associated with an average loss of .5 kg, compared with a .2 kg gain in the placebo group. During six months of treatment with citalopram, the mean weight gain was less than 1 kg; over a one-year period, the mean increase was less than 1.5 kg (
64).
Few data have been reported on the relationship between sertraline and weight change. In one study, patients who were treated with sertraline for six months lost more weight than did those in the control group (
65).
Other antidepressants. Mirtazapine has been associated with an increase in appetite and weight and with dry mouth. The mechanism of this effect is not well understood. An average gain of about 2 kg after six weeks of treatment has been reported (
66). A dose-dependent relationship between mirtazapine and increased appetite and weight has been demonstrated (
57). Mirtazapine is known to enhance norepinephrine and serotonin release, increase serotonin 5-HT
1 function, and block serotonin 5-HT
2 and 5-HT
3 receptors.
Trazodone was studied with 243 patients and was associated with weight gains of up to .5 kg (
53). In another investigation, increases were not observed, and there was a slight weight loss among subjects who were overweight (
65). In contrast, other studies have shown an association of trazodone with weight gain (
57). The drug's mechanism of action is related to inhibition of presynaptic serotonin reuptake, with a possible mild postsynaptic serotonergic agonism.
Bupropion, in a three-month trial with 58 depressed patients, was associated with a mean decrease in weight of 3 kg (
67). Forty-two patients who had complained of increased appetite or weight gain with previous antidepressant therapy lost an average of 4 kg each. Bupropion is a rational choice when stability or loss of weight is important (
14).
Venlafaxine does not appear to cause weight changes (
57,
60). Some evidence suggests that nefazodone may cause weight loss (
60,
68).
Other agents
Benzodiazepines generally do not influence body weight, but alprazolam may be an exception because of associated appetite stimulation (
57,
69). Research has provided evidence of increased food consumption without significant weight gain among women taking alprazolam.
Zaleplon is a nonbenzodiazepine hypnotic drug that may increase both appetite and weight (
57). The same applies to buspirone, an anxiolytic (
57,
60). Few data on weight changes are available for zolpidem and hydroxyzine. Barbiturates have not been associated with weight changes, but few published data are available.
Naltrexone, an opioid antagonist, may cause weight loss (
60). Among alpha
2-adrenergic receptor agonists, clonidine has been associated with usually nonsignificant gains (
57,
60). Beta blockers have not been associated with weight change (
57,
60).
The antiparkinsonian drugs—amantadine, biperidine, diphenhydramine, and trihexyphenidyl—do not interfere with body weight. Benztropine, however, may cause loss of appetite and weight loss (
55). Psychostimulants, such as dextroamphetamine and fenfluramine, have been used in the treatment of obesity (
57). It has not been established whether the weight-loss effects of such medications are due primarily to appetite suppression, other central nervous system actions, or metabolic changes. Nonetheless, this effect could have negative consequences for children in terms of growth suppression (
54,
57). Pemoline and methylphenidate also can contribute to loss of appetite and weight loss, at least transiently (
57).