Violent and criminal behavior are likely related to complex environmental and social circumstances, but heritable factors also have been implicated (
1,
2). The specific neural mechanisms leading to delinquency and impulsive aggression are poorly understood, although they have been the subject of spirited speculation and debate for literally centuries (
2–
4). Arguably, the clearest link between genetic variation and aggression exists for monoamine oxidase A (MAO-A, MIM 309850), a key enzyme in the catabolism of monoamines, especially serotonin. The serotonergic system has been implicated in impulsivity and manifest violent behavior in animals and both auto- and heteroaggression in humans (
2).
MAOA and
-B genes, likely derived from the same ancestral gene, are both located on the X chromosome (Xp11.23), comprising 15 exons with identical intron-exon organization (
5). MAO-A provides the major enzymatic clearing step for serotonin and norepinephrine during brain development, whereas MAO-B activity increases dramatically after birth (
5). Mouse knockouts for
MAOA, but not
MAOB, have elevated brain levels of serotonin, norepinephrine, and dopamine. They show enhanced amygdala-dependent emotional, but not motor, learning (
6), and males exhibit dramatically increased aggressive behavior (
7). In humans, a Dutch kindred with a missense mutation in the
MAOA gene has been described (
8): hemizygous males, representing functional gene knockouts, exhibited a pattern of impulsively violent criminal behavior for generations.
Although functionally disabling variants of the gene are rarities outside of the laboratory setting, a common variable number of tandem repeats polymorphism of the
MAOA gene has been described that strongly impacts transcriptional efficiency: enzyme expression is relatively high for carriers of 3.5 or 4 repeats (
MAOA-H) and lower for carriers of 2, 3, or 5 repeats (
MAOA-L) (
9). Although conflicting evidence exists for the association of genotype with trait impulsivity in human cross-sectional studies, a clear and pronounced gene-by-environment interaction was found in a large longitudinal study of children followed for 25 years in which
MAOA-L predicted violent offenses in males with adverse early experience (maltreatment) (
10). This finding, replicated in the majority of further studies (
11–
13), but not all (
14), suggests a deficiency in the neural systems for emotional regulation and memory as possible substrates for the observed gene-environment interaction, because they are essential for the encoding, retrieval, and extinction of negative emotional information expected to be associated with maltreatment during childhood. This finding agrees with current proposals linking brain structures involved in emotional control, such as amygdala and medial prefrontal and orbitofrontal cortices, to the emergence of violent behavior (
3,
4). However, whereas two previous functional MRI (fMRI) studies suggested an effect of
MAOA genotype during a cognitive task in small samples (
15,
16), no data related to emotion processing or brain structure are available.
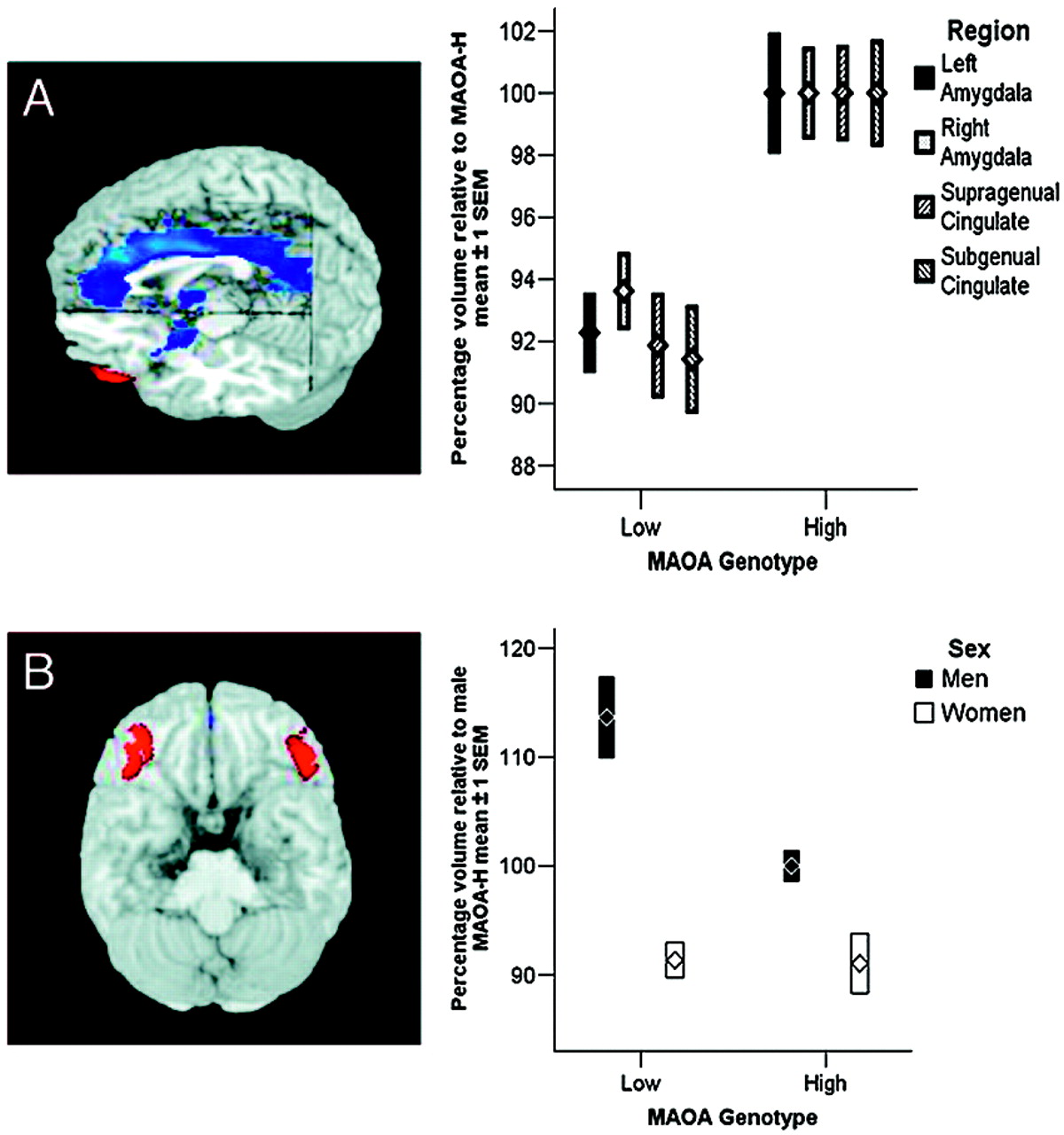
In the present study, we examined a large sample of healthy volunteers (Table 1, which is published as supporting information on the PNAS web site) using a multimodal imaging approach that we have shown previously to be sensitive to genetic variation affecting the serotonergic system (
17). Because our sample was nonviolent, we are not studying the relationship of
MAOA and violence
per se, but rather the effects of one specific genetic factor on relevant aspects of brain circuitry without contamination by other interacting genetic and epidemiological risk factors that may be implicated in the emergence of this complex behavior and that could obscure or exaggerate the genetic effect (e.g., drug or alcohol use or maltreatment) (
1,
2). Voxel-based morphometry was used to canvass the brain for regional volume changes related to genotype (
17), previously seen in genetic variation related to serotonin (
17), a major modulator of neurodevelopment (
18,
19). Three functional magnetic resonance paradigms were used to assess aspects of emotional and cognitive control, subserved by limbic circuitry and conceptually linked to impulse control. To probe circuits of emotional arousal, we used affectively salient social stimuli (angry and fearful faces) previously shown to reliably activate amygdala (
17). To examine the neural circuitry engaged by emotional memory, we used incidental encoding and retrieval of neutral and aversive visual scenes. Finally, because cognitive inhibitory processing has also been implicated as a substrate of impulsivity (
4), we studied cognitive inhibitory control using a no-go variant of the “flanker” task (
20). We hypothesized that carriers of
MAOA-L would exhibit structural and functional changes in brain circuitry subserving these various regulatory functions related to emotion and inhibitory control. Because the behavioral effects of
MAOA variation have been consistently more penetrant in males in both animal (
7) and human (
8) studies, we also expected that some physiological and structural differences would be more pronounced in males than females.
Discussion
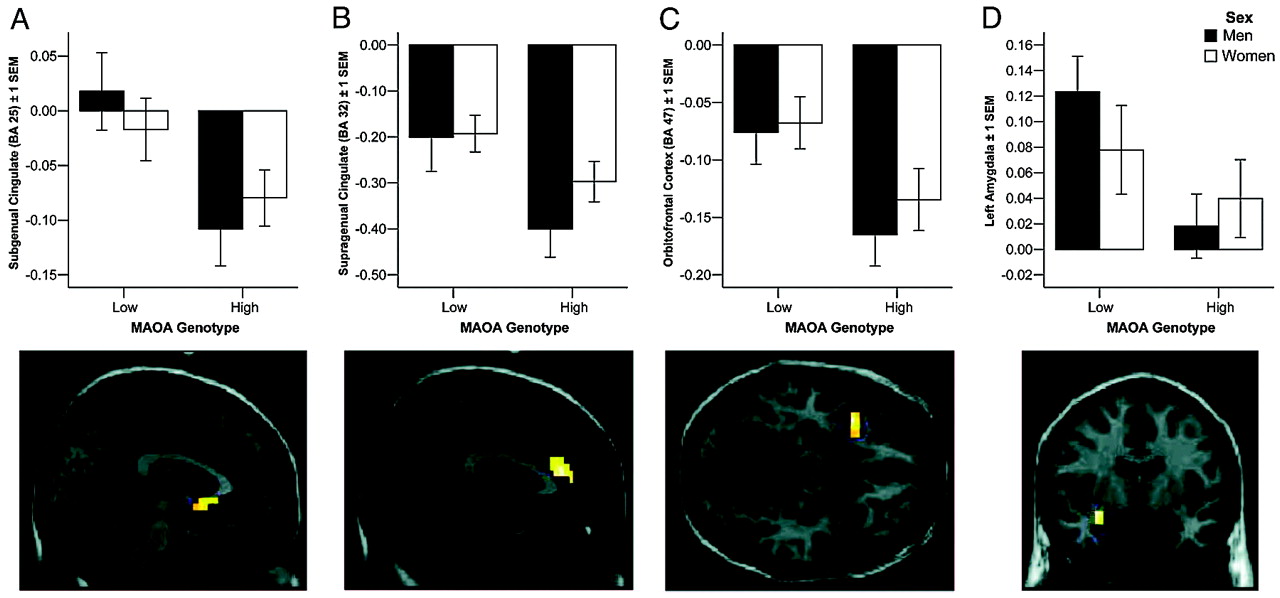
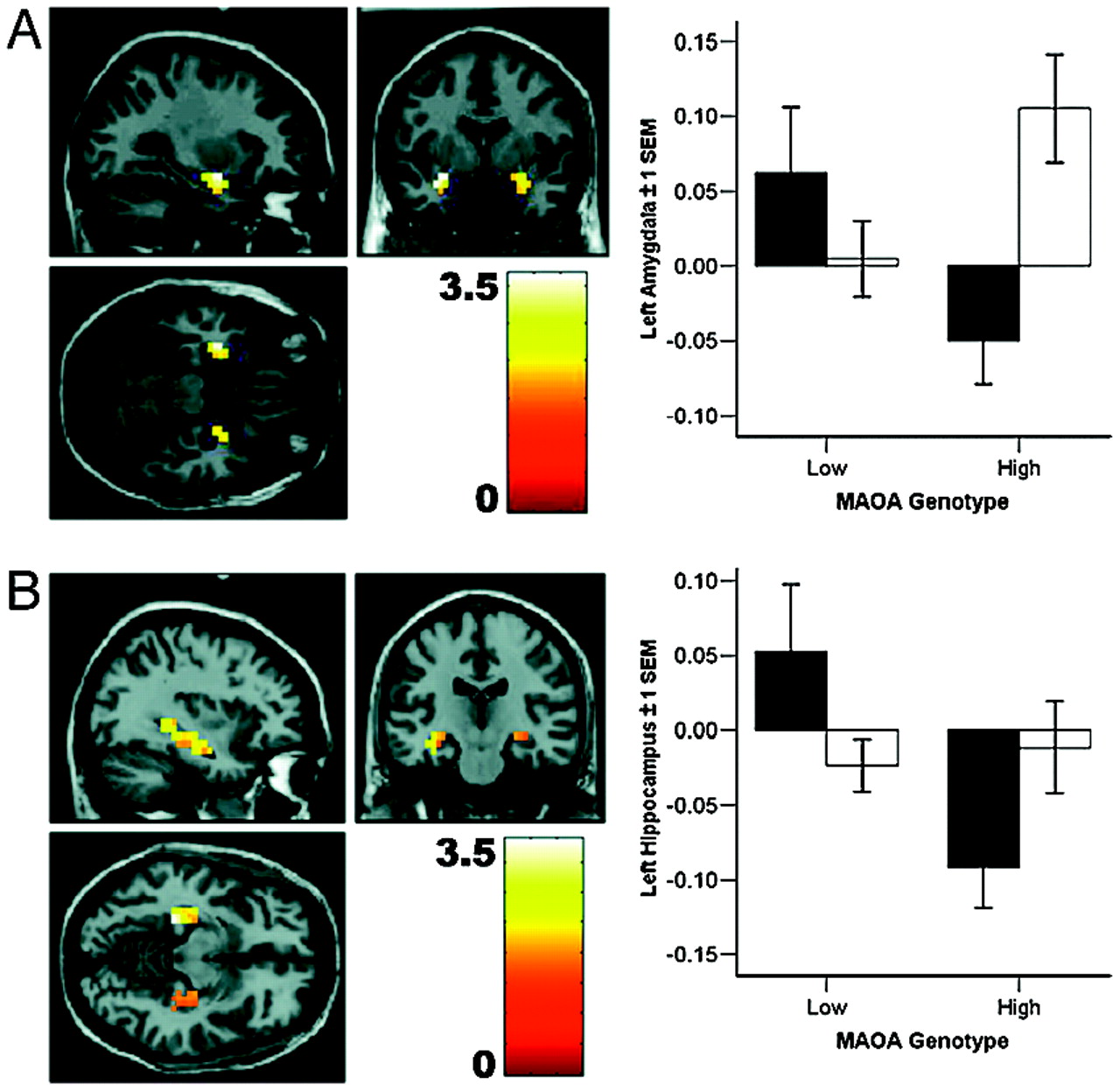
Our analysis revealed pronounced genotype-related structural and functional changes in corti-colimbic circuits previously linked to affect regulation, emotional memory, and impulsivity. Importantly, because our sample was psychiatrically normal, the variation observed is clearly compatible with normal mental health and does not imply or suggest increased risk for violence in our sample. Rather, our data identify neural mechanisms associated with one specific gene epidemiologically associated with risk for violent and impulsive behavior (
10–
13). By itself, this gene is likely to contribute only a small amount of risk in interaction with other genetic, epidemiological, and sociobiographical factors. Of the identified functional differences, increased amygdala activation, in particular, is associated with anger and with perception of angry faces (
22); amygdala stimulation in animals can induce violent behavior; and amygdala ablation has been reported to reduce impulsive violence in humans (
23). Because we used a low-level baseline (fixation) in our imaging tasks to increase reliability of the amygdala response (
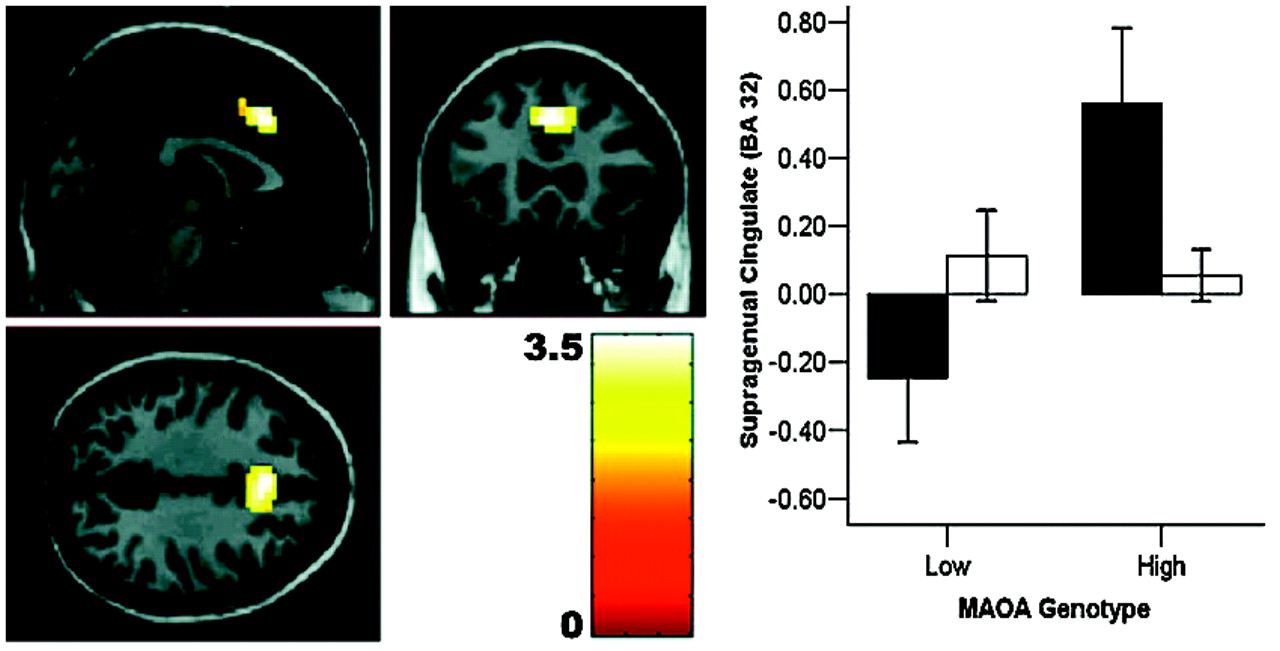
24), our data probe only the overall reactivity of fear-related circuitry to faces; further work using high-level baselines is necessary to implicate processing of specific facial emotions. It is noteworthy that the most robust structural changes were observed in cingulate, the brain region with the highest density of serotonin receptors within the human cortex (
25) and the recipient of dense projections from amygdala (
26). Convergent evidence strongly suggests a key regulatory role for cingulate and medial prefrontal cortices in extinguishing amygdala reactivity and in emotional arousal (
26): ventral cingulate reactivity predicts amygdala signaling during extinction in humans (
21) and lesions of this region markedly impair fear extinction (
27). Given evidence that cingulate modulates amygdala activity by inhibition, our finding of reduced cingulate reactivity in
MAOA-L subjects provides a potential mechanistic account for the observed increased amygdala activity in this group. In this context, it is worth mentioning that animal studies demonstrate a role for serotonergic neurotransmission in modulating the inhibitory functions of cingulate cortex (
28).
Previous work has demonstrated a similar although less statistically robust and more focal effect on amygdala function as a consequence of genetic variation in the serotonin transporter,
5-HTTLPR, (
17,
29); there, as here, it was the genetic variant associated with higher synaptic serotonin levels, presumably during neurodevelopment, that was associated with impaired limbic structure, increased amygdala activation, and relatively decreased response of cingulate circuitry regulating amygdala. Serotonin is further implicated by preclinical data showing enduring anxiety in mice with transient transgenic alterations of serotonin signaling shortly after birth or in mice treated perinatally with serotonin reuptake inhibitors (
19). These results suggest separate genetic mechanisms apparently converging on the development of limbic circuitry, consistent with evidence that 5-HT impacts on neuronal proliferation, migration, differentiation, and synaptogenesis (
18,
19). It is further of relevance to note that abnormal amygdala and orbitofrontal volume, both of which have been linked to deficits in fear perception and social cognition, have been observed in a study of partial deletions on chromosome Xp, including the
MAOA locus (
30).
One consistent finding in research on
MAOA genotype effects on violence and aggression is the more pronounced impact on males. In our analysis of
MAOA-dependent structural changes, we found a significant genotype-by-sex interaction in OFC. In human and animal models, OFC activity has been associated with representation of the relative reward value of primary and secondary (learned) reinforcers (
31). In particular, OFC and OFC-amygdala interactions are critical for stimulus-reinforcement association learning (
31) and have been hypothesized to link sensory representations of stimuli with the social judgements made about them on the basis of their motivational value. Lesions of OFC are associated with disinhibition and antisocial behavior (
3,
4), and we have recently obtained imaging results showing reciprocal regulation of amygdala by both OFC and cingulate (
32), suggesting that OFC provides a layer of control that may be especially important if cingulate function is suggested to be compromised, as here. Furthermore, we found significantly decreased functional connectivity with amygdala in men, indicating that this regulatory mechanism may be intrinsically weaker in men and that the genotype-dependent variation in OFC structure and function may therefore be more likely to result in insufficient amygdala regulation by this route.
When considering potential neurobiological correlates of aggression, it is important to bear in mind that overt behavior is expressed in a complex interaction of biological, psychological, and social determinants (
2). Factor analytic studies of dimensional aspects of human temperament suggest that a distinction can be drawn between so-called impulsive-reactive and instrumental, goal-directed dimensions of aggression (
33), although this distinction is not universally accepted. The instrumental factor has been associated with psychopathy, is often accompanied by diminished empathy and remorse, and has been linked to reduced amygdala activation and OFC volume (
4). The genetic data presented here, which show the opposite effects associated with the risk allele, suggest that these two dimensions may be genetically dissociable; argue against an association of
MAOA genotype with instrumental aggression and for a genetic risk for impulsive violence; and indicate that, whereas both instrumental and impulsive aggression may be present to varying degrees in most violent offenders, the risk imparted by the specific genetic variation studied here contributes to the impulsive dimension of this complex behavior. This finding is in good agreement with the preclinical and human data reviewed above that indicate an association of the serotonergic system with impulsive violence (
2) and may clarify findings from epidemiological studies in which outcome measures such as arrests for violent offenses map ambiguously on these two dimensions of violent behavior (
10).
A striking finding in the
MAOA literature is the mediation of this genotype effect by past environmental adversity, suggesting an impact on brain systems related not only to acute emotional regulation, but also to the processing of emotional experience. Our findings during emotional memory in humans are analogous to enhanced emotional learning observed in
MAOA knockout mice (
6). If replicated, this finding could suggest that heightened sensitivity to adverse experience may underlie the increased vulnerability of
MAOA-L males exposed to abuse during childhood (
10–
13). As observed previously, abnormal cingulate regulation of amygdala could also contribute to this gene-by-environment interaction by impaired extinction of conditioned fear (
17).
Predisposition to impulsive violence by means of abnormal activation and regulation of emotion-related amygdala function might be further enhanced by deficient neural systems for cognitive control (
4), especially over inhibition, the capacity to suppress prepotent but inappropriate behavior (
20) that might originate from a dysregulated affective response. Although the rostral cingulate is key to the regulation of acute affective arousal and emotional learning, inhibitory control of prepotent cognitive responses is thought to be critically dependent on caudal aspects of anterior cingulate (
28,
34). Our study of genetic influences on cognitive impulse control revealed a sex-dependent impairment in precisely this area of cingulate, affecting men only. Our finding of a genotype-by-sex interaction in this region therefore provides a plausible neural mechanism for reduced cognitive inhibitory control in risk allele-carrying males, suggesting synergistic impairment in cognitive and emotional neural regulatory mechanisms that might render
MAOA-L men at especially high risk for a neural phenotype that plausibly relates to the slightly greater probability of impulsive violence.
The cellular mechanism for the observed sex-by-genotype interactions is not currently known. Because we found similar effects for amygdala and cingulate volume and activation for both sexes, a simple gene-load-effect mechanism linked to the localization of
MAOA on the X chromosome seems unlikely. This conclusion was further substantiated by post hoc analysis of the female data, where the functional response of heterozygotes carrying both a high- and a low-expressing allele was intermediate between female homozygotes, and female homozygotes are similar to male hemizygotes (Fig. 6). This correspondence in functional response indicates similar gene dosage in the homo-/hemizygous groups, providing some in vivo evidence for a physiological consequence of X-inactivation in the human brain. X-inactivation, if sufficiently random across cells, would also predict an intermediate response at the neurobiological systems level in female heterozygotes, as was indeed observed. Of note, estrogens affect transcription of
MAOA in brain (
35), and sex hormone receptors are prominently expressed in amygdala, cingulate, and OFC (
36). It should also be borne in mind that, in addition to direct cellular effects of sex, serotonin-related aggression is a complex behavior that manifests in social contexts that are themselves strongly affected by sex; for example, higher dominance status in primates is associated with
MAOA-L and aggression in males (
37), but not females.
Because the variation in
MAOA related to increased serotonin levels has been predominantly associated with impulsive violence, and the analogous allele of
5-HTLPPR with anxiety and depression, it is instructive to compare similarities and differences in the neural mechanisms associated with these genes (
17). Both impact on structure and function of amygdala and perigenual cingulate cortex, indicating a shared mechanism of emotional regulation under serotonergic control and predicting some overlap in clinical association, as is indeed observed (
38). However,
MAOA showed much more extensive effects in both structure and activation, notably affecting more caudal regions of the cingulate associated with cognitive control, as well as OFC and hippocampus. This finding may reflect the broader metabolic effect of variation in MAO-A, which catabolizes not only serotonin, but also other neurotransmitters, notably norepinephrine (
5), which is also implicated in neurodevelopment and emotional experience. We speculate that, whereas our results suggest that a dysregulated and hyperreactive amygdala response contributes to both anxiety and violence (“fight or fright”), the manifestation of violence in behavior may require impairment in additional layers of control, be they emotional (OFC) or cognitive (caudal cingulate). Our genetic results are in good agreement with current hypotheses about the neural substrates of violence (
3,
4). In addition, as discussed, an impact on emotional memory may relate to the pronounced gene-environment interaction observed for
MAOA.
In summary, we present multimodal imaging data delineating functional and structural differences in a prefrontal-amygdala-hippocampal system for emotional regulation, memory, and cognitive control that suggest neural mechanisms for genetic bias toward impulsive violence. This work implicates neural systems for social adaptation and cognition under partial genetic control and suggests adverse consequences for increased serotonergic tone during brain development in humans.