Gender differences in the frequency of affective disorders are well established in the psychiatric literature.
1 Unipolar disorders occur more frequently in women than in men, and it has been proposed that this difference may be due to biological factors. Gender-based differences in brain functioning or organization that may contribute to differences in frequency of affective disorders have been found by some investigators.
2–4 For example, Borod
2 found that facial recognition of emotion is more bilaterally distributed in females than males; Gur et al.
3 have shown that gender differences in resting glucose metabolism in the limbic system may be the basis of some cognitive and emotional differences between sexes; and Shaywitz et al.
4 found that cerebral organization of language was more bilateral in its cerebral localization in females than males.
One might expect, given these gender-based differences in brain organization, that brain injury would affect men and women differently. A common psychopathological manifestation after stroke is mood disorder. Mood disorders following stroke have been proposed as a model to study affective illness in general. If women are biologically more vulnerable to developing a depressive disorder, one might expect that a precipitating factor common to men and women (such as a cerebrovascular accident) would provoke more depression in females than males. One would also predict poststroke depression to be associated with clinical variables indicating a greater biological predisposition in females. On the other hand, depression in males might be associated with nonbiological factors such as severity of physical and psychosocial impairment.
Given these gender-related findings in other patients populations, we undertook a study to investigate the frequency and clinical correlates of acute poststroke depression in women compared with men. We hypothesized that females would have an increased frequency of depression that would be correlated with family and prior personal history of mood disorder, whereas males would have depressions that correlated with severity of physical impairment and social support. To test these hypotheses, we examined depression both as a categorical and a dimensional variable.
RESULTS
Frequency and Severity of Depression
Twenty-one of 170 males (12.3%) and 31 of 131 females (23.6%) had major depression (likelihood ratio chi-square=6.57, df=1, P=0.01). In this analysis, patients with minor depression (males, n=31, 18.2%; females, n=24, 18.3%) were included in the non–major depression group. If minor depressions were excluded, the increased frequency of major depression in females compared with males remained significant.
Male and female patients with major depression had similar mean depression severity scores as measured by the Ham-D scale (males, 16.5±5.93; females, 16.2±5.34).
CT Scan Examination
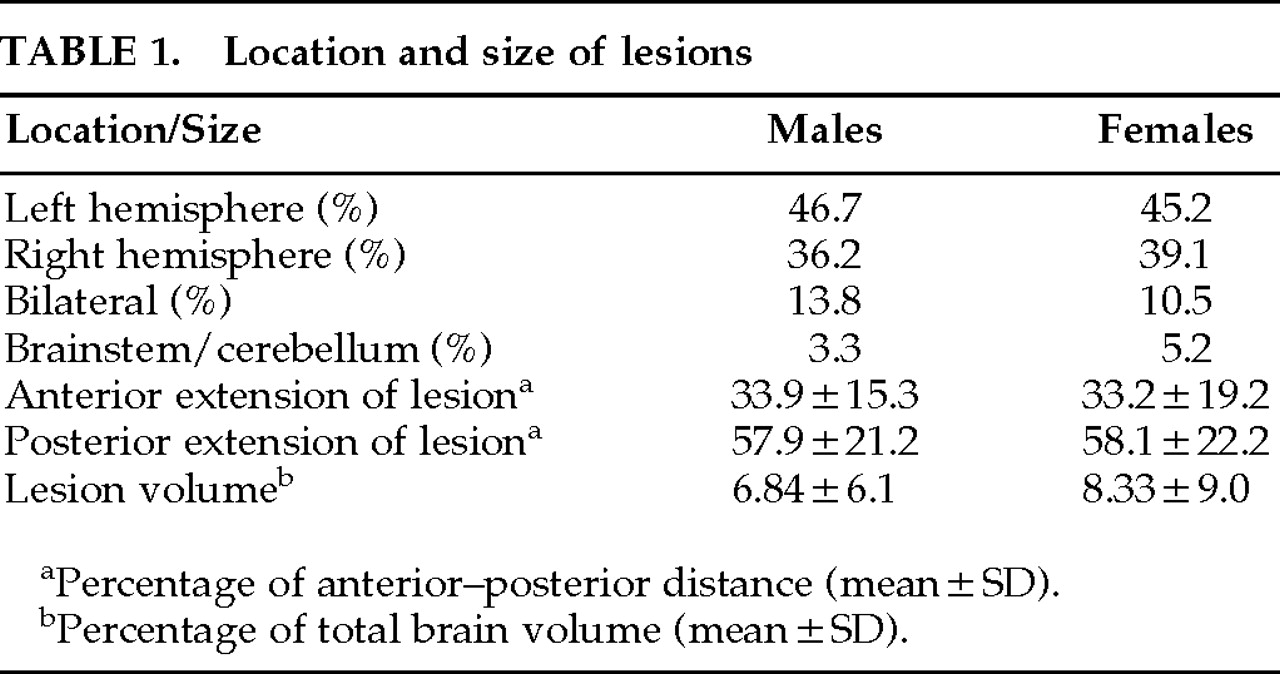
No significant difference was found between male and female patient groups in hemispheric side of lesion or the anterior or posterior extension of lesion along the anterior–posterior axis of the brain and lesion volume (
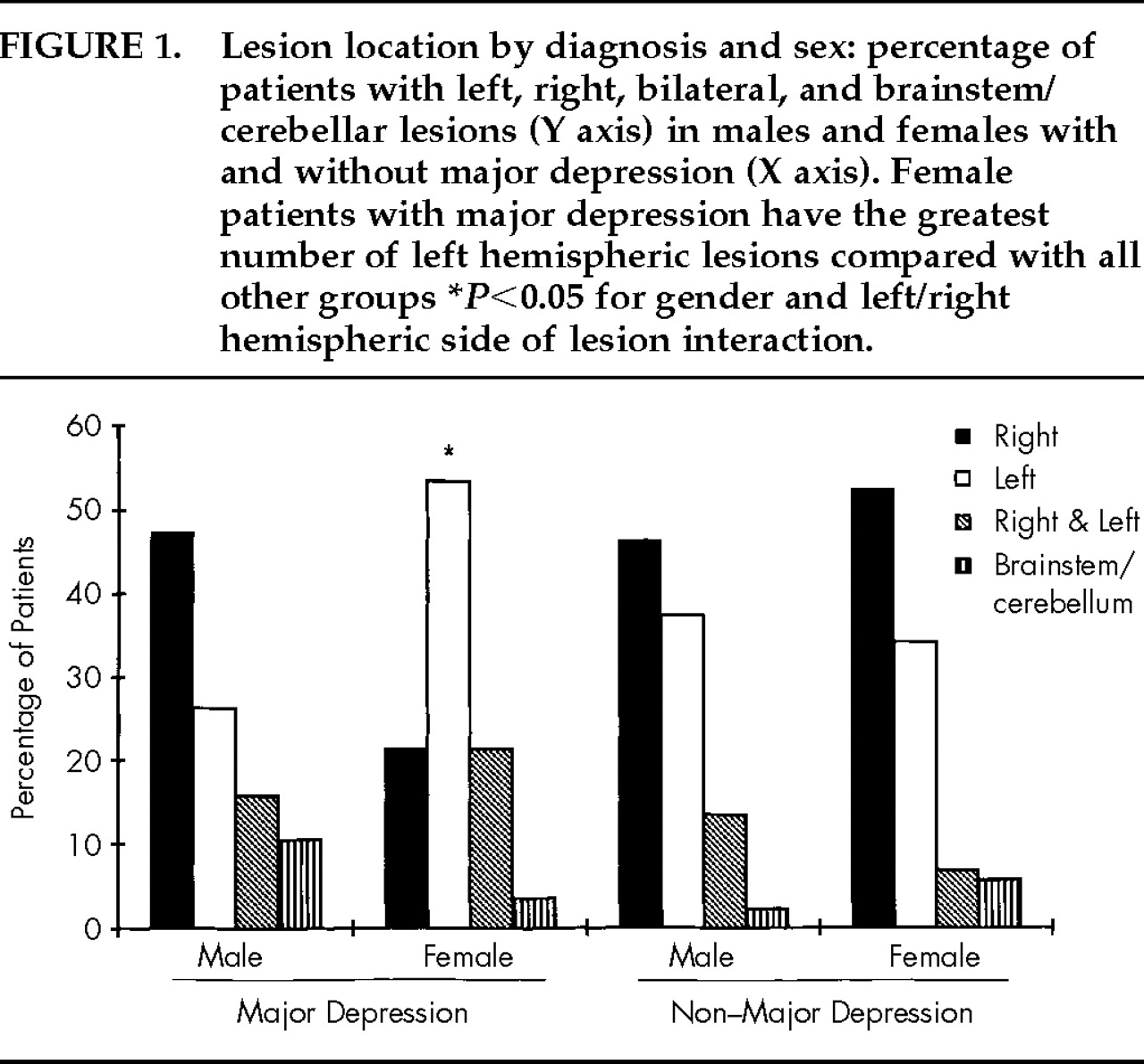
Table 1). However, more depressed women had a left hemisphere lesion than depressed men (
Figure 1). Nominal logistic regression using gender, left and right hemispheric side of lesion, and their interaction yielded a significant overall effect (χ
2=11.8, df=3,
P=0.008) and a significant interaction (Wald χ
2=4.63, df=1,
P=0.031). Models using anterior or posterior extension or volume of lesion and their interactions with gender did not show significant effects.
Relationship to Background and Clinical Characteristics
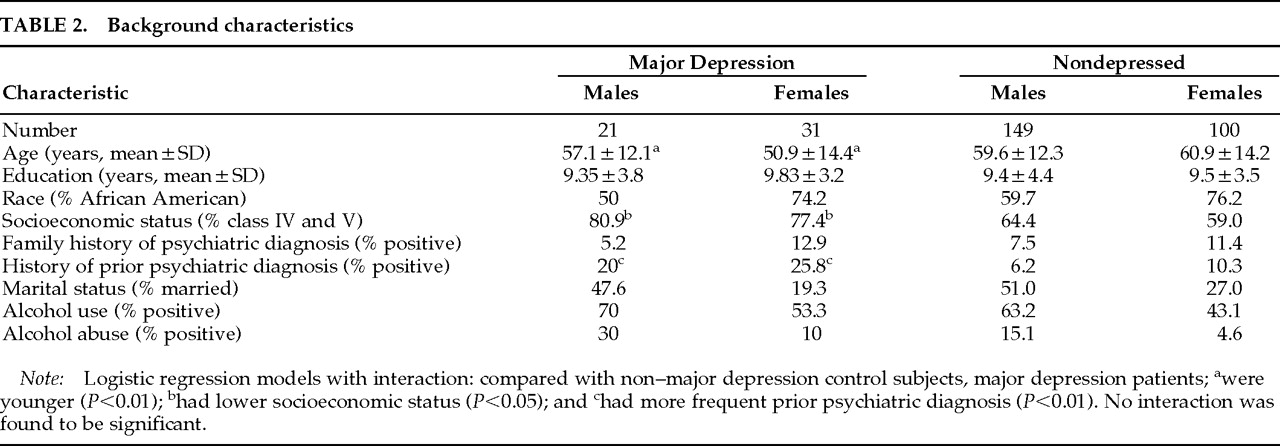
Background characteristics of subjects are shown in
Table 2. Because the association of female gender was with major and not with minor depression, background characteristics were compared between major depression and non–major depression patients. Logistic regression analyses of gender and each background characteristic and their interaction on major depression diagnosis (categorical variable) were performed with and without inclusion of minor depression patients in the non–major depression group. Major depression patients were younger (age effect,
F=6.87, df=1,287,
P=0.008), had a greater frequency of lower socioeconomic status (Wald χ
2=5.25, df=1,
P=0.021), and had a greater frequency of prior psychiatric diagnosis (Wald χ
2=8.29, df=1,
P=0.004). A trend effect for alcohol abuse was also found (Wald χ
2=3.17, df=1,
P=0.07). No background characteristics×gender interaction showed a significant effect. When minor depression patients were excluded from the analysis, results were essentially the same.
We then investigated the unique predictors of depressive symptom severity (depression as a dimensional variable). We used age, race, education, prior and family psychiatric history, socioeconomic status, marital status, alcohol use, and alcohol abuse as the independent variables and the Ham-D as the dependent variable in two separate stepwise (backward) regression analyses for male and female patients. Analyses revealed that younger age in males (F=6.07, df=9,85, P=0.016) and prior psychiatric diagnosis in females (F=5.80, df=9,60, P=0.019) predicted severity of depressive symptoms. In females, younger age showed a nonsignificant trend (F=2.73, df=9,60, P=0.10).
Cognitive Impairment, Activities of Daily Living, and Psychosocial Functioning
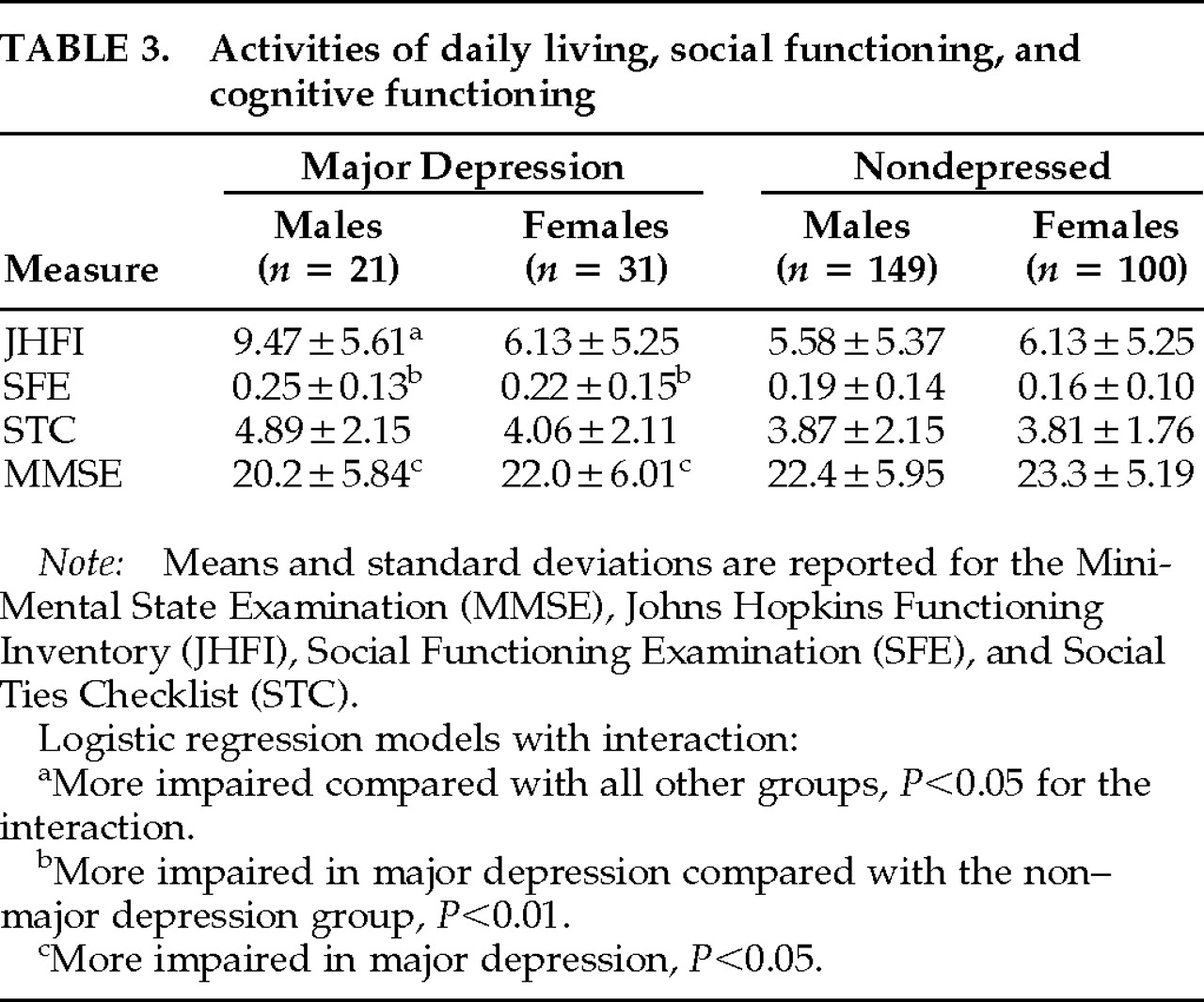
Data concerning activities of daily living, social impairment, and cognitive impairment are shown in
Table 3. Nominal logistic regression of gender and JHFI on major depression diagnosis yielded a significant overall effect (χ
2=14.25, df=3,
P=0.002). There were significant gender (Wald χ
2=9.12, df=1,
P=0.002), JHFI (Wald χ
2=4.10, df=1,
P=0.042), and interaction effects (Wald χ
2=4.10, df=1,
P=0.042). Regression of gender and STC on major depression diagnosis yielded a significant overall effect (χ
2=11.93, df=3,
P=0.007) and a significant gender effect (Wald χ
2=3.98, df=1,
P=0.046), but no STC (Wald χ
2=3.19, df=1,
P=0.07) or interaction effects (Wald χ
2=0.73, df=1,
P=0.4). Regression of gender and SFE on major depression diagnosis yielded a significant overall effect (χ
2=11.61, df=3,
P=0.008) and a significant SFE effect (Wald χ
2=7.45, df=1,
P=0.006), but no gender (Wald χ
2=1.06, df=1,
P=0.3) or interaction effects (Wald χ
2=0.05, df=1,
P=0.8). Regression of gender and MMSE on major depression diagnosis yielded a significant overall effect (χ
2=8.27, df=3,
P=0.040) and a significant MMSE effect (Wald χ
2=3.87, df=1,
P=0.049), but no gender (Wald χ
2=0.11, df=1,
P=0.7) or interaction effects (Wald χ
2=0.07, df=1,
P=0.7). Hence, an increased risk of depression was associated with female gender interacting with impairment in activities of daily living performance, so that among males, those with the greatest physical impairment were the most depressed. Depression in both males and females was associated with impaired social functioning and greater cognitive impairment.
We then used depression as a dimensional variable (Ham-D) and conducted stepwise (backward) multiple regressions. The analyses revealed that activities of daily living (JHFI: F ratio=13.4, df=4,112, P=0.0004) and social functioning (SFE: F ratio=4.29, df=4,112, P=0.040) predicted severity of depressive symptoms in males. Among the measures of cognitive and social functioning, the only predictor for severity of depression in females was MMSE (F ratio=4.63, df=4,87, P=0.034).
In summary, activities of daily living and social impairment predicted severity of depression in males. Prior psychiatric diagnosis and cognitive impairment predicted severity of depressive symptoms in females.
DISCUSSION
Several interesting findings emerged from this study. First, major depressive disorder after stroke was twice as frequent among females as among males. Second, depression was significantly associated with left hemisphere lesion location only among women. Third, among women the risk factors for depression were younger age, personal history of psychiatric disorder, and cognitive impairment, whereas among men, the risk factors for depression were younger age and impairment in activities of daily living and social functioning.
Before we discuss the implications of these findings, several limitations of the study should be acknowledged. First, most patients enrolled in this study were African Americans of lower socioeconomic status. Thus, our conclusion about gender differences may apply only to this population. Second, patients with significant comprehension deficits were excluded. Thus, we do not know if comprehension impairment affected depression frequency differentially in males and females. Third, this study examined patients during the acute poststroke period. We do not know if gender differences with regard to depression changed over time.
Results from this study show that, as in primary depression, there is greater frequency of poststroke depression in females compared with males. The most fundamental and important question raised by this study is why major depression is more common in women than in men. Although this study is unable to definitively answer this question (the question remains unanswered for functional depression as well), the association of increased depression with prior psychiatric history suggests a predisposition for depression in females. These results are in agreement with those of Burvill et al.,
20 who reported that poststroke depression in females was frequently associated with a history of depressive disorder prior to stroke. A biological vulnerability for depression in females is suggested also by the finding that only women showed a significant association of major depression with left hemisphere lesion. A cerebrovascular injury may have acted either as a stressor or a biological trigger for major depression.
Over the last 15 years, we
19,21 and other groups
22,23 have shown that major depression following brain injury is more frequent after left anterior lesions. The results of this study may help to explain some of the inconsistent past results
24–26 with regard to poststroke depression and lesion location. Major depression has also been shown to be associated with cognitive impairment,
15,19 but this finding has not always been replicated.
27 The results from this study may also shed some light on these inconsistencies. However, since the cognitive impairment in this study was ascertained by using the MMSE, a screening test that assesses predominantly left hemisphere cognitive function, we are not able to say whether in females, cognitive impairment is directly associated with greater depression or is a consequence of the greater frequency of left hemispheric lesions in females with major depression. The latter interpretation, however, contrasts with the report that cognitive impairment assessed by using a large neuropsychological battery in subjects with left hemispheric stroke and depression encompasses functions not restricted to the left hemisphere.
28 These issues will require further studies.
Males appeared to be more susceptible to developing a depressive response to physical and social impairment. This finding replicates the results of Morris et al.
5 They found a greater frequency of depression among men with greater physical disability than among men with less severe impairment. Other studies
29–31 reported an association of depression and functional impairment, but no gender-based analyses were performed. Our findings also confirm those of Morris et al.,
5 who reported that depressed men tended to perceive their social support as less adequate compared with nondepressed men. Åström et al.
23 showed that “living alone” was associated with depression at initial evaluation, but they did not perform gender-based analyses.
The association of social impairment with depression in males may apply to other medically ill populations.
6,7 In a sample composed predominantly of men with traumatic brain injury, we found that poor social functioning was the clinical variable that was most consistently associated with major depression throughout 1-year follow-up.
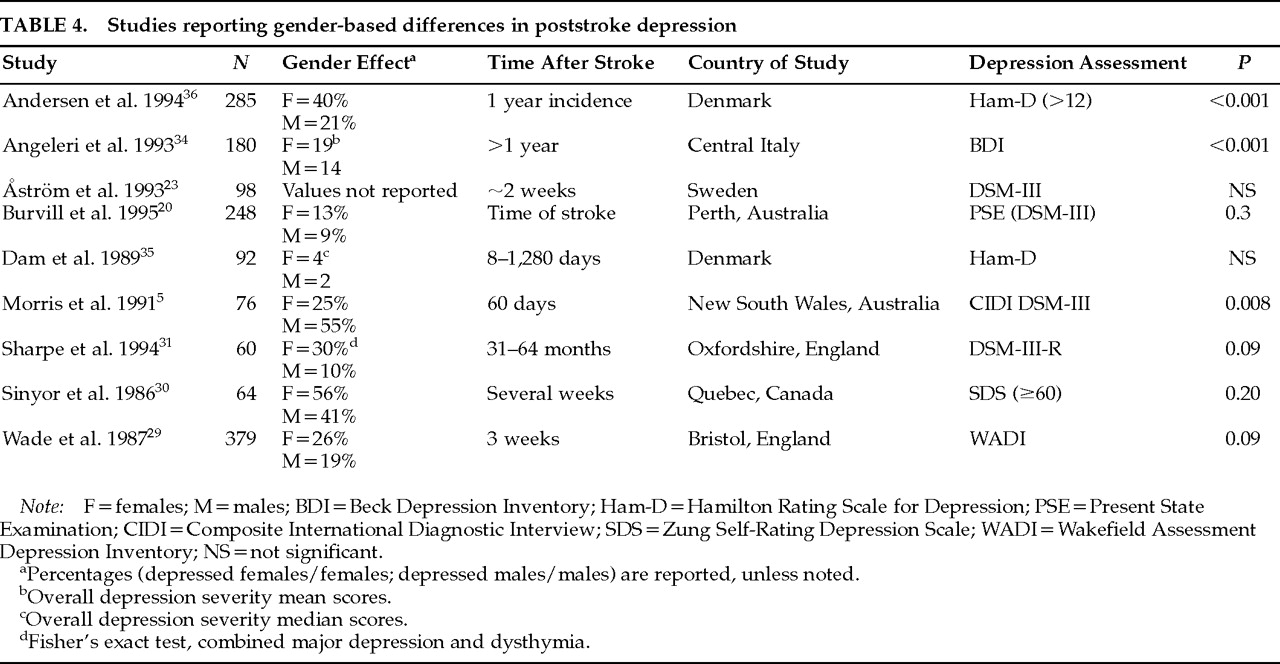
32,33Previous studies examining the prevalence of major depression among males and females have not found consistent results (
Table 4). There are several possible explanations for this lack of consistency, including differences in diagnosis, selection criteria, time of evaluation, ethnicity, and socioeconomic status. As shown in
Table 4, it may be any single factor or, more likely, a combination of them that contributes to the lack of consistency among studies.
One of the reasons that studies may differ in the prevalence of gender-related major depression is the method of diagnosis. The criteria used to identify subjects with poststroke depression often differ from study to study. Use of depression scales and a depression cutoff score versus semistructured interviews and diagnostic criteria, or self-rating versus observer-rated scales, is likely to bear a significant impact on results. Similarly, since the increased frequency of depression in females is a phenomenon that is restricted to major depression, grouping minor and major depression under a common rubric may also affect the results.
Selection criteria may be another important cause of differences in findings concerning frequencies of males versus females with major depression. Excluding patients with a previous history of psychiatric disorder may mask gender differences in the prevalence of major depression.
30 Morris et al.,
5 in the only study that reported increased prevalence of depressed males, included patients only if they were able to “nominate a person they could turn to for support following the stroke.”
Time of evaluation is another reason why some studies may have failed to identify differences in the frequency of major depression in males versus females. Burvill at al.
20 reported that 13% of female patients and 9% of males were depressed at the time of stroke but that the prevalence of depression at 1 year was almost equal between sexes (about 24%). Among those patients who were depressed at 4 months, significantly more women (39%) than men (16%) had been depressed at the time of the index episode.
20 This example shows how time from index stroke to evaluation, as well as setting (hospital versus rehabilitation facility or nursing home), may also be a cause for discrepant results.
Population differences in ethnicity and socioeconomic status may also account for result inconsistencies in gender-related differences in poststroke depression.
5,20 A longitudinal study conducted in Sweden
23 did not find female gender to be associated with major depression. Two studies conducted in England showed an association of major depression with female gender (when other clinical and pathological variables were controlled)
31 and an association of female gender with greater depression (assessed with the Wakefield Assessment Depression Inventory).
29 Angeleri et al.,
34 studying 180 consecutive patients in central Italy, also reported greater severity of depression (assessed with the Beck Depression Inventory) in females. Although Dam et al.,
35 studying a sample of young and only slightly disabled patients in Glostrup, Denmark, found a similar trend of twice as severe depression measured by the Ham-D among women, this result did not reach statistical significance. Another study conducted in Denmark (Aalborg/Farsø) reported that the 1-year incidence of depression in an unselected stroke population was 85/209 (41%), with 60% of the depressed patients being female.
36 The percentage of depressed females that the authors report is exactly comparable to ours (depressed males=20, 39%; depressed females=31, 61%).
Further investigations of these interesting gender-related differences are clearly needed.
In summary, major depressive disorder in the acute poststroke period is more frequent in females (at least within the type of population studied here) than males. In addition, poststroke depression has different correlates and predictors in males compared with females. This result may be the effect of psychosocial factors, different biological predisposition, or both. These findings might also have important therapeutic implications. Perhaps men would be more likely to respond to physical therapy for activities of daily living or social intervention, while women might be more likely to respond to psychological or somatic therapy.